Infant feeding in the first months/years of life affects the health in the short and long term. Breast milk is the perfect food due to its many benefits. However, when breastfeeding is not possible, infant formulas are the best alternative. The aim of this study is to define the role of supplemented formulas in infant nutrition using the opinion of a panel of experts in infant gastroenterology and nutrition.
Material and methodsA survey, consisting of 62 items divided into 5 blocks, was completed by 48 panelists using the Delphi method to achieve a professional criteria consensus on nutritional aspects of infant formulas.
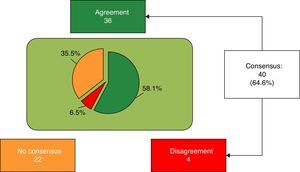
ResultsConsensus was reached on 64.6% of the items, although opinions were divided on the nutritional aspects of infant formulas, and their influence on body and brain development and immune maturity.
ConclusionsThe experts polled reached consensus on the suitable composition of lipids, lactose, calcium, vitamin D, and prebiotics in infant formulas, for a correct cerebral, immune and somatic development. There was no consensus on ill-defined topics, such as nutritional quality of proteins, use of thickeners, taurine supplementation, probiotic, and symbiotic aspects. More studies are necessary to confirm these issues.
La alimentación infantil en los primeros meses/años de vida condiciona la salud a corto y largo plazo. La lactancia materna es la alimentación ideal por sus innumerables beneficios. Sin embargo, cuando no es posible la alimentación con leche materna, las fórmulas infantiles constituyen la mejor alternativa. El objetivo del estudio fue definir el papel de las fórmulas de inicio y continuación para lactantes mediante la opinión de un panel de expertos en gastroenterología y nutrición infantil.
Material y métodosEncuesta realizada mediante el método Delphi por 48 especialistas en pediatría y nutrición infantil. El cuestionario constaba de 62 ítems, estratificados en 5 bloques, sobre aspectos nutricionales de las fórmulas infantiles.
ResultadosSe consensuó el 64,6% de los ítems, estableciéndose un acuerdo no unificado respecto a aspectos nutricionales y su impacto sobre el desarrollo corporal, cerebral y la maduración inmune.
ConclusionesSegún los expertos encuestados, existe consenso sobre la adecuada composición en lípidos, lactosa, calcio, vitamina D y prebióticos, de las fórmulas infantiles, para el correcto desarrollo cerebral, inmunitario y somático. No hubo consenso en aspectos aún no bien definidos, como la calidad nutricional de las proteínas, la utilización de espesantes y la suplementación con taurina, probióticos y simbióticos. Son necesarios más estudios que determinen estos aspectos.
Child feeding in the first months/years of life conditions to a great extent the child's health in the short and long term.1 The World Health Organisation recommends exclusively breastfeeding for the first 6 months of life, afterwards supplemented with proper feeding until at least 2 years of age.2 Breastfeeding is ideal due to innumerable benefits: it provides protection against infections, promotes neurodevelopment and reduces blood pressure/cholesterol and later risk of suffering from diabetes/obesity.1 However, there are circumstances under which it is necessary to supplement or replace breastfeeding.
During the first years of life, a high percentage of children are fed with infant formulas. The United States Food and Drug Administration estimates than 40, 50 and >75% of babies receive infant formulas at 3, 6 and 12 months, respectively.3
To get as close as possible to breast milk, in the last decade infant formulas have been supplemented with functional ingredients: polyunsaturated fatty acids, beta-palmitate, nucleotides, prebiotics, probiotics, symbiotic, carnitine, taurine, etc., thus turning these formulas into “functional foods.”
Various studies have attempted to determine the clinical relevance and safety of supplements. Most have been funded by child nutrition companies, and include few subjects with a short follow-up or somewhat inconsistent methodology.4 Therefore, aside from their nutritional effects, the beneficial effects of infant formulas on allergies, lactose intolerance, respiratory/digestive infections, infant colic, hypercholesterolaemia, etc., are still unclear. In the case of supplementation with prebiotics and/or probiotics, the Nutrition Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition considers it necessary to carry out more studies to define the optimal amounts, recommended duration, potential long-term risks/benefits, the use in special populations, etc.4,5
Due to these uncertainties, and the widespread use of these formulas, it is important to clarify some aspects relating to their quality, safety and benefits (short/long term). The purpose of this study is to ascertain the opinion of child gastroenterology and nutrition experts on certain controversial aspects of infant formulas, especially in areas with insufficient scientific studies. The degree of consensus will allow us to identify whether a uniform criterion for action exists, and detect areas for improvement.
Material and methodsA modified, two-round Delphi method was used in this study, consisting of a structured professional consensus technique, which has all the advantages of the original technique,6 but none of its main disadvantages.
The project consisted of 4 phases: (1) creation of a scientific committee (n=5) to steer the project; (2) selection of a panel of experts (48 paediatricians) in child nutrition and gastroenterology; (3) preparation and sending an electronic survey in 2 rounds; and (4) analysis and assessment of results and preparation of conclusions.
A technical team directed and supervised the process and carried out the instrumental implementation.
The survey (62 items formulated as a statement [affirmative/negative] including a professional criterion) contained 5 blocks: (1) nutritional and digestive aspects (25 items: 7 General, 3 Fats, 3 Carbohydrates, 7 Proteins and 5 Digestive formulas); (2) immune system, brain development and retina (9 items); (3) bone health (12 items: 4 Vitamin D and 8 Calcium); (4) prebiotics (7 items); and (5) probiotics (9 items).
The items were assessed using a 9-point ordinal Likert-type scale.
The study was undertaken from July to September 2013.
To analyse the opinion of the group and the consensus reached, median scores and the concordance level reached were used.7 The items were considered as consensus when there was concordance: less than one third of respondents rated items outside the 3-point region ([1-3] - [4-6] - 7-9]) containing the median. In these cases, the value of the median determined the group consensus: disagreement if the median was ≤3, or agreement if the median was ≥7. The items where the value of the median was in the [4-6] region were considered doubtful.
Disagreement was established when the scores for a third or more of the respondents were in the [1-3] region, and for another third or more in the [7-9] region.
The items where no concordance or disagreement were observed were considered undetermined.
Items with no consensus (doubtful, with disagreement or uncertain consensus) were reconsidered in the second round, together with those with high dispersion and interquartile range ≥4 points (scores between the 25th and 75th percentile).
The results of the second round were analysed following the same criteria.
To graphically compare the items, the average score of the respondents was calculated with their 95% confidence interval. The closer the mean of an item was to 1 or 9, the higher the consensus in agreement or disagreement, respectively.
A lower confidence interval was interpreted as a higher agreement. Items with no consensus were analysed descriptively to determine whether this was due to persisting disagreement over a criterion, or the majority positioning of the panel in the region of doubt about the item (the majority of the group with no final criterion; region [4-6]).
The result of each item is presented indicating the mean, the median, the percentage of distribution of respondents outside the region of the median, the interquartile range, and the result of the consensus (Tables 1–5).
Results obtained from Block 1: nutritional and digestive aspects.
| Items | Mean | Median | Percentage outside the median | Interquartile range | Result |
|---|---|---|---|---|---|
| General | |||||
| 1. Exclusive breastfeeding during the first 6 months of life is enough for proper growth in healthy infants | 8.65 | 9 | 1.75 | 0 | Agreement |
| 2. Initiation formulas are indicated in infants from the first day up to 4–6 months who cannot benefit from breast milk | 8.54 | 9 | 3.51 | 0.5 | Agreement |
| 3. Follow-on formulas are indicated up to 2 years | 5.4 | 7 | 47.92 | 4 | No consensus |
| 4. Growth formulas or 3-milks are for children over 2 years of age | 4.63 | 5 | 87.5 | 4 | No consensus |
| 5. Growth formulas or 3-milks are for children from 12 months of age upwards | 6.48 | 7 | 29.17 | 2 | Agreement |
| 6. In children aged 1–3 years old, growth milks ensure a lower risk of renal overload of solutes | 6.40 | 7 | 31.25 | 1 | Agreement |
| 7. Growth milks contain more saturated fats and cholesterol than cow's milk | 7.28 | 8 | 15.8 | 2 | Agreement |
| Fats | |||||
| 8. The fat content of initiation formulas is based on vegetable oils with no proper profile for infants | 3.35 | 3 | 25 | 1 | Disagreement |
| 9. The addition of beta-palmitate in initiation formulas, similar to breast milk, leads to less formation of insoluble calcium soaps, which decreases the hardness of faeces and, therefore, improves constipation | 7.67 | 8 | 8.77 | 2 | Agreement |
| 10. Initiation formulas must have a high proportion (>40%) of palmitate in 2 or beta position | 6.88 | 7 | 14.6 | 0 | Agreement |
| Carbohydrates | |||||
| 11. The reduced lactose content of an initiation formula is useful to avoid infant colics | 3.17 | 3 | 20.8 | 0.5 | Disagreement |
| 12. The lactose of infant formulas facilitates the absorption of calcium | 7.74 | 8 | 12.3 | 2 | Agreement |
| 13. Replacing part of the lactose of infant formulas with glucose or dextrin maltose polymers improves their digestibility | 6.11 | 7 | 27.7 | 1 | Agreement |
| Proteins | |||||
| 14. Initiation infant formulas must contain a 40/60 proportion of casein/seroproteins | 7.77 | 8 | 10.5 | 2 | Agreement |
| 15. The high protein content of infant formulas leads to a higher risk of obesity in the adult age | 6.85 | 7 | 16.7 | 1 | Agreement |
| 16. The use of partially hydrolysed proteins in initiation formulas increases their digestibility | 6.46 | 7 | 28.1 | 2 | Agreement |
| 17. Partially hydrolysed proteins are useful in the prevention of infant colics | 4.71 | 5 | 83.3 | 5 | No consensus |
| 18. The use of formulas with partially hydrolysed proteins is adequate for infants who cannot be exclusively fed with breast milk | 2.93 | 2 | 24.6 | 2.5 | Disagreement |
| 19. Supplementing breast milk with a bottle of adapted (whole) or partially hydrolyzed milk is enough to induce an allergic sensitisation | 7.32 | 8 | 17.5 | 2 | Agreement |
| 20. The complete proteins of infant formulas (initiation and follow-on) have a better nutritional quality than partially hydrolysed proteins | 3.54 | 3 | 29.2 | 2.5 | Disagreement |
| Digestive formulas | |||||
| 21. The addition of thickeners to initiation formulas is useful in infants with gastroesophageal reflux | 5 | 6 | 83.3 | 4 | No consensus |
| 22. Anti-regurgitation formulas decrease the number and intensity of regurgitations in “physiological” gastroesophageal reflux | 6.56 | 7 | 14.6 | 0 | Agreement |
| 23. The addition of medium-chain triglycerides to digestive or “comfort” formulas improves absorption and the symptoms of infant colics | 5.5 | 7 | 41.7 | 4 | No consensus |
| 24. Anti-regurgitation formulas do not alter the bioavailability of calcium and oligo elements | 5.56 | 5.5 | 64.6 | 2 | No consensus |
| 25. Anti-constipation formulas are efficient for the prevention and treatment of functional constipation of infants, by being supplemented with palmitic acid in beta position, GOS and nucleotides | 6.6 | 7 | 26.3 | 2 | Agreement |
GOS, galactooligosacarides.
Results obtained from Block 2: immune system, brain development and retina.
| Items | Mean | Median | Percentage outside the median | Interquartile range | Result |
|---|---|---|---|---|---|
| 26. Docosahexaenoic acid is essential for correct development of the nervous system in term infants, and its supplementation in initiation formulas should be compulsory | 6.79 | 7 | 31.6 | 2 | Agreement |
| 27. Arachidonic acid is essential for correct development of the nervous system in term infants, and its supplementation in initiation formulas should be compulsory | 6.94 | 7 | 18.8 | 0 | Agreement |
| 28. The addition of n-3 LCPUFA without n-6 is prejudicial for the weight to height ratio development of infants fed with initiation formula | 4.52 | 5 | 41.7 | 2 | No consensus |
| 29. The addition of LCPUFA is not necessary, provided n-6 (linoleic acid) and n-3 (alpha-linoleic acid) content is adequate | 5.38 | 5 | 56.3 | 2 | No consensus |
| 30. Bovine lactoferrin has important antioxidant and antimicrobial effects and, therefore, its supplementation in formulas must be compulsory | 5.29 | 5 | 56.3 | 2.5 | No consensus |
| 31. Taurine has important effects on the development of vision and auditory systems and, therefore, its supplementation must be compulsory in initiation formulas for healthy term infants. | 6.42 | 7 | 35.4 | 1 | No consensus |
| 32. Taurine has important effects in the development of vision and auditory systems and, therefore, its supplementation must only be compulsory in initiation formulas for healthy, low birth-weight term infants | 4.88 | 5 | 79.2 | 4 | No consensus |
| 33. Nucleotides have important effects on the maturation of the immune system and the prevention of diarrhoea and, therefore, their supplementation in initiation formulas must be compulsory | 6.48 | 7 | 31.3 | 1 | Agreement |
| 34. Nucleotides have effects on somatic and cephalic growth, which is associated with brain development; therefore, their supplementation must be compulsory in initiation formulas | 6.27 | 7 | 35.4 | 2 | No consensus |
LCPUFA, long-chain polyunsaturated fatty acids.
Results obtained from Block 3: bone health.
| Items | Mean | Median | Percentage outside the median | Interquartile range | Result |
|---|---|---|---|---|---|
| Vitamin D | |||||
| 35. Vitamin D supplements must be administered to infants younger than one year fed with infant formulas | 7.13 | 7 | 20.8 | 2 | Agreement |
| 36. Premature infants need more vitamin D than term infants | 6.91 | 8 | 22.8 | 2 | Agreement |
| 37. High-risk infants (with allergy to proteins, vaccines, or with multi-allergies) at risk for vitamin D deficiency must receive 400IU/day of vitamin D supplement, regardless of blood vitamin D levels | 6.42 | 7 | 27.1 | 1.5 | Agreement |
| 38. Infants fed with exclusive breastfeeding must be supplemented with vitamin D | 7.53 | 8 | 17.5 | 2 | Agreement |
| Calcium | |||||
| 39. The bioavailability of breast milk calcium is higher than that of infant formulas | 8.09 | 8 | 8.77 | 2 | Agreement |
| 40. The contribution of calcium in artificial formulas must be similar to that of breast milk | 5.4 | 7 | 45.8 | 4 | No consensus |
| 41. The bioavailability of calcium is similar in dairy products and fortified foods | 4.6 | 5 | 52.1 | 3 | No consensus |
| 42. Children with lactose intolerance (and receiving formula with no lactose as the only milk contribution) need supplementation with oral calcium | 4.29 | 3 | 35.4 | 3.5 | No consensus |
| 43. Children with epilepsy receiving treatment with anticonvulsant medication have a special risk of osteopenia and osteoporosis, and therefore must be supplemented with calcium and vitamin D systematically | 5.94 | 6 | 50 | 2 | No consensus |
| 44. We must recommend the use of growth formulas as milk content in children 1–3 years, due to its higher calcium content than cow's milk | 4.88 | 5 | 79.2 | 4 | No consensus |
| 45. In patients with vitamin D deficit there must be simultaneous supplementation with calcium | 6.38 | 7 | 25 | 0.5 | Agreement |
| 46. For a better absorption of calcium it is important that the Ca/P ratio is not below 1 or above 2 | 7.75 | 8 | 7.02 | 2 | Agreement |
Results obtained from Block 4: Prebiotics.
| Items | Mean | Median | Percentage outside the median | Interquartile range | Result |
|---|---|---|---|---|---|
| Probiotics | |||||
| 47. The bifidogenic effect of prebiotics, as with probiotics, depends on the dose | 6.96 | 7 | 16.67 | 0 | Agreement |
| 48. Prebiotics added to milk formulas are useful in the prevention of allergies in infants with risk of atopic disease | 5.48 | 7 | 45.83 | 3.5 | No consensus |
| 49. Prebiotic-enriched formulas have a beneficial effect on infants with constipation | 6.79 | 7 | 29.82 | 2 | Agreement |
| 50. Prebiotics-enriched formulas decrease episodes of diarrhoea in infants | 6.44 | 7 | 27.08 | 1.5 | Agreement |
| 51. The addition of GOS in infant formulas improves the development of the intestinal flora (intestinal microbiota) and the immune system | 7.19 | 7 | 17.54 | 1 | Agreement |
| 52. The addition of GOS/FOS in a fixed amount is convenient to improve the development of the microbiota intestinal flora of the infant | 6.96 | 7 | 24.56 | 1 | Agreement |
| 53. The new formulas containing 100% of GOS are much more beneficial for the health of the infant | 4.88 | 5 | 35.42 | 0 | No consensus |
FOS, fructooligosacarids; GOS, galactooligosacarids.
Results obtained from Block 5: Probiotics.
| Items | Mean | Median | Percentage outside the median | Interquartile range | Result |
|---|---|---|---|---|---|
| Probiotics | |||||
| 54. The use of formulas with probiotics is safe in large premature babies | 5.33 | 5 | 56.25 | 2.5 | No consensus |
| 55. The use of formulas containing probiotics in large premature babies reduces the incidence of necrotising enterocolitis and nosocomial sepsis | 6.56 | 7 | 25 | 0.5 | Agreement |
| 56. In infant formulas supplemented with probiotics, the proper strain must be selected | 8.35 | 9 | 3.51 | 1 | Agreement |
| 57. Probiotic strains that are not inactivated by heat must be selected, since their effect would be lost when heating the milk formula | 7.91 | 8 | 10.5 | 2 | Agreement |
| 58. The administration of a symbiotic in milk formulas may be more beneficial than administration of pre or probiotics alone | 6.44 | 7 | 35.4 | 3 | No consensus |
| 59. Supplementation of infant formulas with probiotics may be useful in the prevention of minor digestive problems, such as infant colics | 6.44 | 7 | 25 | 1 | Agreement |
| 60. The use of probiotics in infant formulas is beneficial for infants with constipation | 6.29 | 7 | 35.4 | 1.5 | No consensus |
| 61. The supplementation of infant formulas with probiotics may prevent the development of allergies | 6.52 | 7 | 29.2 | 1.5 | Agreement |
| 62. The Nutrition Committee of the ESPGHAN does not recommend the “routine” use of probiotics in initiation and follow-on formulas | 8.21 | 9 | 6.25 | 1 | Agreement |
ESPGHAN, European Society for Paediatric Gastroenterology, Hepatology and Nutrition.
To justify the agreement or disagreement reached on aspects evaluated in each item, a literature search was carried out, using, among other tools, the PubMed platform. The purpose was to identify published evidence regarding each of the statements (clinical practice, research, etc.).
ResultsIn the first round, 20 of 62 items were agreed. Those with no agreement (Block 1: 3-6, 8, 10, 11, 13, 15, 17 and 20-24; Block 2: 27-34; Block 3: 35, 37, 40-45; Block 4: 47, 48, 50, 53; Block 5: 55 and 58-62) were proposed for reconsideration in the second round, with 20 agreed. The 22 (35.4%) with no agreement were: Block 1: 3, 4, 17, 21, 23 and 24; Block 2: 28-32 and 34; Block 3: 40-44; Block 4: 48 and 53; Block 5: 54, 58 and 60.
Finally, 40 of 62 items (64.6%) were agreed, 36 (58.1%) in agreement and the remaining 4 (6.5%) in disagreement with the statement (Fig. 1). Block 1, Nutritional and digestive aspects: 19 of 25 items were agreed (5 of subsection General, 6 of subsection Fats and 9 of subsection Proteins), 15 in terms of agreement and 4 in disagreement. The 6 remaining items were not agreed (Table 1). Block 2, immune system, brain development and retina: 3 of 9 items were agreed, all in agreement; the remaining 6 were not agreed (Table 2). Block 3, bone health: 7 of 12 items were agreed (4 of subsection Vitamin D and 3 of subsection Calcium) (Table 3); the remaining 5 were not agreed. Block 4, prebiotics: 5 of 7 items were agreed, all with agreement; the other 2 items were not agreed (Table 4). Block 5, probiotics: 6 of 9 items were agreed in terms of agreement, and the remaining 3 were not agreed (Table 5).
DiscussionThe opinion expressed by child nutrition and gastroenterology experts showed the lack of consensus among professionals with regard to infant formulas; only 65% of the statements were agreed.
Echoing the literature,2,8 it was agreed that breast milk allows for proper growth of healthy infants during the first 6 months of life. If breastfeeding is not possible, there was consensus regarding the validity of initiation formulas up to 4–6 months, when the slow transition to diversified feeding takes place.9 There was no agreement on the correct age for starting continuation milks, probably due to the various compositions required according to the development stage.10 However, for growth formulas (modified follow-on formulas)11 their validity was agreed after one year of age, showing a knowledge of their nutritional characteristics (correct lipid profile)11 and the lack of legislation on the subject.
As regards the protein content of infant formulas, experts did not agree that whole formulas are more nutritional than partially hydrolysed formulations; however, there was no agreement on the validity of the latter in infants not fed with breast milk. The casein/seroprotein 40/60 proportion, which has an amino acid content similar to that of breast milk,9 was considered correct. It was also agreed that a high protein content increases the risk of obesity, showing knowledge on that subject, as described above.12
No consensus was reached as regards the role of partially hydrolysed proteins and the reduction of lactose in formulas to prevent infant colic. Colic is assesed through the baby's crying time; adding hydrolysed casein to the infant formula does not provide sufficient evidence of reduction,13 and neither do lactose-reduced formulas13 or supplements of medium-chain triglycerides.14
Regarding the onset of allergies, panelists showed agreement that there are no differences between formulas with complete or partially hydrolysed proteins. The role of proteins in the prevention of allergies remains unclear.15
There was no consensus on the effect of additional thickeners in initiation formula on the infant's gastroesophageal reflux, although it was agreed that they decrease the number and intensity of physiological regurgitations. Although they reduce regurgitation episodes, thickeners are moderately effective in healthy infants,16 which is why they are not to be indicated routinely. There was no agreement that anti-regurgitation formulas alter the bioavailability of calcium and oligo elements, although Horvath et al. showed that thickeners may alter the absorption of calcium, other ions and carbohydrates.16
There is agreement in the sense that the bioavailability of calcium is higher in breast milk in relation to infant formulas,17 but not as regards the correct content of calcium in the formulas. This discrepancy might be explained by the fact that the highest concentrations of calcium in infant formulas are related to a lower absorption and bioavailability of calcium; in formulas with calcium concentrations of 389, 659 and 1024mg/L, the absorption fraction is 57, 47 and 37%, respectively.17 It is established that the proportion of Ca/P must not be below 1 or above 2.8
There was also agreement regarding the fact that the addition of lactose facilitates the absorption of calcium and the development of bacterial microflora.18 However, the increase in cases of lactose intolerance justifies agreement to replace part of the lactose with glucose/dextrin maltose to improve digestibility. In formulas with no lactose, it has been shown that even if the absorption of calcium is lower than those with lactose, it is enough to meet infants’ needs,17 provided intake satisfies daily recommendations. There was no agreement on whether the infants fed with formulas with no lactose need calcium supplements because this is still a controversial issue.
There was agreement regarding the need for vitamin D supplements in children younger than one year of age. Similarly, it was agreed that premature babies are at higher risk of vitamin D deficiency due to the lower intake of milk and the presence of low levels of that vitamin on birth. There are studies indicating that they do not require a higher intake of vitamin D than non-premature babies19; high doses of this vitamin do not improve bone mineralisation, and therefore the advice is to administer 400IU/day as for the other newborn.20 The consensus may be related to knowledge of the recommendations of vitamin D intake (according to the Nutrition Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition and the Spanish Society of Paediatric Gastroenterology, Hepatology and Nutrition).
There was no consensus on systematic supplementation with calcium and vitamin D in epileptic children with anticonvulsive medication. It appears that paediatric gastroenterologists are aware of the risk of changes in the calcium–phosphorus metabolism in these patients (in the case of anticonvulsive medication, immobilisation). This is not the case with most paediatrician neurologists who do not perform a routine assessment of bone health.21 Although some recommend this supplement to improve bone structure and quality of life,22 others consider that this contribution must be assessed in conjunction with other factors (nutritional intake and sun exposure).23
There was agreement that the addition of a suitable proportion of beta-palmitate reduces the formation of insoluble calcium soaps and reduces constipation. Beta-palmitate, in the various formulas, simulates breast milk by increasing absorption of fatty acids and calcium,24 in addition to benefiting bacterial microflora.25 As regards the taurine amino acid, there was no consensus on its implementation, despite its association with neuronal development and cellular differentiation-migration in the cerebellum and visual cortex.26 The obligation to add taurine was not agreed, neither for healthy term infants or low-weight infants. A meta-analysis did not show statistical differences in the growth of infants supplemented with or without taurine.27 There was also no agreement on compulsory supplementation with bovine lactoferrin; despite the benefits described in paediatric populations (respiratory diseases and haematocrit levels), more studies are necessary to corroborate these effects.28
Regarding supplementation with long-chain polyunsaturated fatty acids (LCPUFA), experts agreed on the benefit of adding docosahexaenoic acid and araquidonic acid (LCPUFA n3 and n6, respectively) for the development of the nervous system. The presence of araquidonic acid and docosahexaenoic acid in infant formulas29 in equal proportions to breast milk has proven to be relevant and safe in achieving proper cognitive development.30 The panelists did not reach an agreement on the prejudicial effect of adding series n3 LCPUFA (with no associated n6) on weight-to-height ratio development. There was also no agreement on the need to add other LCPUFA to formulas containing only essential linoleic and alpha-linoleic fatty acids.
In the case of supplementation of initiation formulas with nucleotides, the experts echoed published findings and agreed that they are necessary due to their effects on the maturation of the immune system and the prevention of diarrhoea, the latter to benefit growth.31 However, no agreement was reached on their effect on brain development.
On the role of prebiotics in infant formulas, there was consensus on their dose-dependent effect32 and that administration increases the number of bifidobacteria in faeces, with positive effect on their consistency. Dairy formulas with prebiotics can have a beneficial effect on infants with constipation, reducing episodes of diarrhoea33 and preventing allergies.34 More research is necessary before using them routinely to prevent allergies.35
The addition of GOS to infant formulas benefits the intestinal flora36 and the immune system.32 The addition of GOS/FOS in a fixed amount improves the development of the intestinal microbiota. The European Commission directive on infant formulas and follow-on formulas (Directive 141/2006, dated December 22, 2006) confirms that FOS and GOS can be added to formulas, provided their content does not exceed 0.8g/100ml, in a combination of 90% oligogalactosyl-lactose and 10% high molecular weight oligofructosyl-saccarose.32
Despite the evidence that probiotics can be used safely in premature babies, according to the Nutrition Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (2011)4 and the Cochrane review of 2011,37 proving their benefits in preventing necrotising enterocolitis in large premature babies, there are concerns for their long-term safety and effects. This may explain the lack of consensus on this issue.
There is a lack of consensus as regards the benefit of the administration of a symbiotic in relation to pre or probiotics, although symbiotic would provide more benefits.38 Very few studies have confirmed this benefit, which is why there was no agreement among panellists.
Several studies have shown the benefits of probiotics in the treatment of colic, but there are hardly any studies on the subject of probiotics in the formulas.39 Most respondents are in agreement on the usefulness of probiotics in colic, which may indicate that the proven benefit of the direct use of probiotics has been extrapolated to their supplementation in infant milks. Thus, L. reuteri has shown certain benefit as treatment of colic in infants exclusively fed with breast milk, but the benefit does not seem so clear if they are fed with formula.40 It has no proven benefit in the prevention of allergies when administered independently.
In conclusion, the results of this study show the agreement of specialists on the validity of breast milk for proper growth of the infant, and that of initiation formulas up to 4–6 months, if breastfeeding is not possible. The correct age to begin growth formulas was established as one year, but the correct age to begin follow-on milks was not established. The proper lipid profile of infant formulas was agreed through the contribution of vegetable fats and beta-palmitate, in addition to the beneficial effect on the gastrointestinal status of the infant. However, there was no agreement on the nutritional quality of complete proteins vs partially hydrolysed proteins, both considered implicated in the onset of allergies, or that thickeners modify the bioavailability of calcium and other oligo elements. As regards lactose, due to the increase in intolerance, specialists considered it appropriate to replace part of this with glucose or dextrin maltose to improve digestibility, despite its capacity to facilitate absorption of calcium. There was also evidence of agreement on the incorporation of docosahexaenoic acid and arachidonic acid, together with nucleotides due to its effect on maturation of the immune system; and supplementation with vitamin D (400IU/day) for all infants, with no agreement on the latter for epileptic children. However, there was no consensus on supplementation with taurine and lactoferrin. Supplementation with prebiotics and GOS/GOF was agreed as advisable for their beneficial effects on constipation, diarrhoea and their immunomodulation potential. However, the lack of studies on the benefits of probiotics and symbiotics justifies the decision that their use is not recommended and the consensus was not to recommend its addition until there are more studies to prove their efficacy.
Conflict of interestThe authors declare that there are no conflicts of interest.
Josefa Barrio Torres.
Juan José Díaz Martín.
Ignacio Manrique Martínez.
Benjamín Martín Martínez.
Eduardo Ortega Páez.
1. Guillermo Álvarez Calatayud.
2. M. Luisa Arroba Basanta.
3. Alfonso Barrio Merino.
4. Patricia Barros García.
5. Luis Carlos Blesa Baviera.
6. Carlos Bousoño García.
7. M. Ángeles Calzado Agrasot.
8. Alejandro Canals Baeza.
9. Francisco Cañabate Reche.
10. Margarita Cañellas Fuster.
11. Esperanza Castejón Ponce.
12. M. Teresa Cenarro Guerrero.
13. Agustín de la Mano Hernández.
14. Beatriz Espín Jaime.
15. Gonzalo Galicia Poblet.
16. Salvador García Calatayud.
17. Carmen García Rebollar.
18. Juan José Gilbert Pérez.
19. M. Ángeles Gómez Ortigosa.
20. Rafael González de Caldas Marchal.
21. Iñaki Irastorza Terradillos.
22. Ana Isabel Jiménez Moya.
23. Carmen Jovaní Casano.
24. M. José López Liñán.
25. Óscar Manrique Moral.
26. Manuel Martín González.
27. Emilio Martín Orte.
28. Eva Martínez-Ojinaga Nodal.
29. M. Lluïsa Masiques Mas.
30. Silvia M. Meavilla Olivas.
31. Antonio Millán Jiménez.
32. Carmen Miranda Cid.
33. Rosa Ana Muñoz Codoceo.
34. Víctor Manuel Navas López.
35. José M. Olivares Rossell.
36. Luis Peña Quintana.
37. Margarita Revenga Parra.
38. M. del Carmen Rivero de la Rosa.
39. Carmen Rodríguez López.
40. Alejandro Rodríguez Martínez.
41. Julio Romero González.
42. Antonio Rosell Camps.
43. César Sánchez Sánchez.
44. Félix Sánchez-Valverde Visus.
45. Alfonso Jesús Solar Boga.
46. Pilar Valverde Viu.
47. Florencia Inés Venturini-
48. Isidro Vitoria Miñana.
Please cite this article as: Barrio J, Díaz-Martín JJ, Manrique I, Martín Martínez B, Ortega E. Consenso experto sobre los aspectos nutricionales de las leches infantiles de inicio y continuación. An Pediatr (Barc). 2015;83:376–386.