There have been very few studies on the effect of assisted electronic prescription on paediatric patient safety. The objective of this study is to compare medication errors that occurred before and after its introduction in a tertiary hospital.
Materials and methodsA quasi-experimental comparative study of medication errors detected before and after assisted electronic prescription introduction. All treatment lines were analysed in order to detect the point in the chain where the medication error occurred, as well as its type and cause. A Delphi study was conducted on the importance of each medication error involving doctors, nurses and pharmacists.
ResultsThe study included 166 patients (83 at each stage). At least one medication error was detected in 92% in the pre-introduction phase patients (2.8 ± 2.1 errors/patient) and 7.2% of post-introduction phase patients (0.1 ± 0.4 errors/patient). The assisted electronic prescription led to an absolute risk reduction of 40% (95% confidence interval = 35.6–44.4%). The main cause of error was lapses and carelessness in both stages. Medication errors were considered serious in 9.5% of cases in the pre-introduction phase, while all of them were mild or moderate in the post-introduction phase.
ConclusionsThe assisted electronic prescription implementation with prescription, validation and medication administration assistance systems significantly reduces medication errors and eliminates serious errors.
El efecto de la implantación de la prescripción electrónica asistida en la seguridad de los pacientes pediátricos ha sido poco estudiado. El objetivo de este estudio es comparar los errores de medicación antes y después de su implantación en un hospital terciario.
Material y métodosEstudio cuasiexperimental comparativo de los errores de medicación detectados, antes y después, de la implantación de la prescripción electrónica. Se analizaron todas las líneas de tratamiento y se recogió el punto de la cadena donde ocurrió el error, el tipo de error y su causa. Se realizó un estudio Delphi sobre la importancia de cada error en el que participaron médicos, enfermeros y farmacéuticos.
ResultadosSe incluyeron 166 pacientes (83 en cada etapa). Se detectó algún error en el 92% de los pacientes en la etapa preimplantacional (2,8 ± 2,1 errores/paciente) y en el 7,2% en la etapa postimplantacional (0,1 ± 0,4 errores/paciente). La prescripción electrónica asistida supuso una reducción absoluta del riesgo de error de un 40% (95% intervalo de confianza = 35,6 - 44,4%). Los lapsus/despistes fueron la principal causa de error en ambos grupos. En la etapa preimplantacional se consideraron graves el 9,5% de los errores y, en la etapa postimplantacional, todos fueron leves o moderados.
ConclusionesLa implantación de la prescripción electrónica con sistemas de ayuda a la prescripción, validación y administración de medicamentos reduce de forma significativa los errores de medicación y elimina los errores graves.
The National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) defines medication error (ME) as “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer. Such events may be related to professional practice, health care products, procedures and systems, including errors related to prescribing, order communication, product labelling, packaging and nomenclature, compounding, dispensing, distribution, administration, education, monitoring, and use.”1
The National Study on Adverse Events related to Hospitalization (ENEAS, for its initials in Spanish), sponsored by the Ministry of Health and Consumption of Spain in 2005, revealed that 37.4% of adverse events were directly related to the medication and 34.8% were preventable.2 The characteristics of the paediatric population (ongoing development, pharmacokinetic and pharmacodynamic peculiarities, the lack of available paediatric drugs that leads to dose calculation and adjustment based on weight and body surface area) and the off-label use of drugs not supported by evidence from clinical trials render this population particularly vulnerable to MEs, with a risk that is 3-fold that in adults.3–5
The introduction of electronic prescribing with decision support systems is recommended by different organizations, societies and expert groups to increase safety in the use of medicines (Institute for Safe Medication Practices [ISMP], American Society of Health-System Pharmacists [ASHP]).6 Several studies support the effectiveness of assisted electronic prescribing (AEP) in reducing the incidence of MEs, although there is significant variation in their results (20–80%),7–10 and this is one of the objectives listed in the position document of the National Health System describing its strategy for patient safety and promotion of safe medication use for the 2015–2020 period.11
Few studies have assessed the implementation of AEP in paediatric patients. We designed this study with the aim of assessing the impact of the introduction of AEP on the frequency of MEs in the department of paediatrics of a tertiary care hospital.
Material and methodsWe conducted a quasi-experimental study in which we analysed drug prescriptions and medication records in the period preceding (retrospective data collection) and the period following (prospective data collection) the introduction of AEP in paediatric care.
We analysed the same months (November-December) in 2015 and 2018 to ensure that the type of diseases and medication used would be similar. We included all patients aged 2 months to 15 years admitted to an inpatient ward with a length of stay of at least 24 h that received active pharmacological treatment (we excluded patients admitted to the intensive care unit because that unit has its own prescription programme).
We analysed all pharmacological treatment regimens prescribed to the patients and created a database (Microsoft Access® 2019) to record the point in the chain where the error happened, the type of error and its cause. Table 1 presents the variables under study.
Variables explored in the analysis of each medication error, both in manual and in assisted electronic prescribing.
| Source of ME |
| Prescription, transcription or validation |
| Types of ME |
| Patient identification errors |
| Missing date or signature of prescribing clinician; illegible signature of prescribing clinician; name of patient or medical record number missing or incomplete in the prescription order or transcript |
| Improper drug selection |
| Medication contraindicated for patient due to a history of allergy |
| Errors in frequency/administration schedule |
| Greater or lesser frequency than appropriate, frequency not specified or expressed in a confusing and/or ambiguous manner |
| Dosing errors |
| Dose greater or lesser than appropriate, dosing not specified or illegible, ambiguous or confusing |
| Wrong duration of treatment |
| Duration greater or lesser than appropriate, incorrect calculation of administration dates |
| Errors in the route of delivery |
| Wrong route, route not specified or illegible in prescription |
| Wrong dosage form |
| Duplication |
| Drug interactions |
| Undertreatment due to error in the interpretation of prescribed treatment |
| Selection of the wrong active ingredient due to confusion, selection of inadequate speciality or medication, omission in the prescription transcript or illegible or confusing prescription information in the prescription order and/or nursing chart |
| Causes of ME |
| Lack of knowledge or training on the drug |
| Lack of knowledge or information on the patient |
| Errors in technology |
| Lapse/slip |
AEP, assisted electronic prescribing; ME, medication error; MP, manual prescribing.
The first period corresponded to the manual prescribing (MP) period and documentation of administration by nurses on paper-based charts. For this period, we performed a manual review of the paper-based medical records, including the prescription order and the transcription by the nurse. We collected data on the errors, omissions, discrepancies and opportunities for improvement in regard to patient management.
In the second period following the introduction of AEP and the electronic documentation of the administration of medication, we collected data prospectively, with the pharmacist actively involved in the verification of the prescriptions made by clinicians.
We conducted an efficacy analysis by comparing the frequency of MEs in the pre-implementation period and the post-implementation period. We classified MEs based on the categories established by the NCC MERP and their adaptation and subsequent update by the Ruiz-Jarabo 2000 working group.12
After, we rated the seriousness of the detected MEs through a Delphi process with participation of 3 doctors, 3 nurses and 3 pharmacists. We selected the members of this expert panel based on their knowledge and experience in the field of paediatrics. The aim of the Delphi process was to reach unanimous consensus in the categorization of the seriousness of the different errors detected in each period. Each error was categorised as mild, moderate or severe. We carried out several consultation rounds in which the categorizations given to each error and their rationales were shared with all members, until consensus was reached in the categorization of the seriousness of each error.13
Based on the decrease in MEs reported in previous studies, we estimated that a sample size of 83 patients in each study period would allow us to detect a significant difference in the frequency of MEs of 20% or greater with a confidence of 95% and a power of 80% assuming a proportion of attrition/missing data of 10%. Given an average of 17 discharges per week, we estimated that we would need to collect data for 5 weeks in each period.
We performed a descriptive analysis of all the variables documented in the study before and after implementation of AEP. We have expressed numerical variables as mean ± standard deviation and interquartile range, and qualitative variables as absolute frequencies and percentages.
We performed an efficacy analysis comparing the percentage of MEs in the MP period and 3 years after the introduction of AEP using the Chi-Square Test or the Fisher's exact test of independence. We calculated the absolute and relative decrease in the risk of error between the 2 periods with the corresponding confidence intervals (CIs). We compared quantitative data between the 2 periods using the -Student's T-Test for independent samples or the Mann-Whitney U test for nonparametric data as applicable.
To analyse the correlation between the use of AEP and the reduction in errors, we fit a multivariate logistic regression model to correct for potential confounders.
All statistical tests were two-tailed, and we considered the result statistically significant if the P-value was less than .05. We performed the statistical analysis with the software SPSS, version 23.0.
The study adhered to the principles of the Declaration of Helsinki and standards of good clinical practice. Before starting the data collection, we obtained the approval of the competent Research Ethics Committee.
ResultsThe study included a total of 166 patients (83 each period). In the pre-implementation period, we only included 75 of the 83 patients in the analysis due to missing data in the paper-based health records in 8 of the patients. Table 2 summarises the baseline characteristics of the patients.
Descriptive analysis of patients in the 2 groups under study.
| Variable | Pre-implementation period (75 patients) | Post-implementation period (83 patients) | P |
|---|---|---|---|
| Age (years), median (interquartile range) | 5.2 (0−15) | 5.7 (0−15) | – |
| Sex, n (%) | |||
| Male | 60 (80) | 50 (60.2) | .044 |
| Female | 15 (20) | 33 (39.8) | |
| Type of patient, n (%) | |||
| Physician | 35 (46.7) | 36 (43.4) | .678 |
| Surgeon | 40 (53.3) | 47 (56.6) | .678 |
| Length of stay, n (%) | |||
| < 7 days | 64 (85.3) | 71 (85.5) | .970 |
| ≥ 7 days | 11 (14.7) | 12 (14.5) | .970 |
| Length of stay, mean ± standard deviation | 4.8 ± 5.6 | 4.8 ± 3.7 | – |
We analysed a total of 1017 prescribed treatment regimens: 510 in 75 patients in the pre-implementation period and 507 in 83 patients in the post-implementation period. The mean number of treatment regimens per patient in the pre-implementation period was 6.8 ± 4.6 (interquartile range, 1−30), compared to 6.1 ± 3.5 in the post-implementation period (interquartile range = 1−18).
We detected some form of error in 92.0% of patients in the pre-implementation period and in 7.2% of patients in the post-implementation period. In the pre-implementation period, we detected a ME in 212 prescribed regimens corresponding to 69 patients, with a mean of 2.8 ± 2.1 errors per patient (interquartile range = 0−12). After the introduction of AEP, we found MEs in 8 prescribed regimens corresponding to 6 patients, with a mean of 0.1 ± 0.4 errors per patient (interquartile range, 0−3). The difference in the number of errors per patient between periods was statistically significant (P < .001). The difference in the number of prescribed regimens with an error (41.6% versus 1.6%) was also statistically significant (P < .001). Table 3 presents the distribution and characteristics of detected MEs.
Distribution y characteristics de los medication errors in the 2 periods.
| Variable | Pre-implementation period | Post-implementation period | P |
|---|---|---|---|
| Reviewed prescribed regimens, n | 510 | 507 | – |
| Prescribed regimens per patient, mean ± standard deviation (IQR) | 6.8 ± 4.6 (1−30) | 6.1 ± 3.5 (1−18) | – |
| Regimens with errors, n (%) | 212 (41.6) | 8 (1.6) | < .001 |
| Patients with at least 1 ME, n (%) | 69 (92) | 6 (7.2) | < .001 |
| MEs per patient, mean ± standard deviation (IQR) | 2.8 ± 2.1 (0−12) | 0.1 ± 0.4 (0−3) | < .001 |
| Source of ME, n (%) | |||
| Prescription | 125 (59) | 4 (50) | .721 |
| Transcription | 87 (41) | 0 (0) | .023 |
| Verification by pharmacist | 0 (0) | 4 (50) | < .001 |
| Types of ME, n (%) | |||
| Errors in identification | 68 (32.1) | 0 (0) | .054 |
| Other errors | 144 (67.9) | 8 (100) | .054 |
| Cause of ME, n (%) | |||
| Insufficient information about the drug | 14 (6.6) | 3 (37.5) | .012 |
| Insufficient information about the patient | 1 (0.5) | 0 (0) | .003 |
| Incorrect use of technology | 0 (0) | 1 (12.5) | .036 |
| Lapse/slip | 197 (92.9) | 4 (50) | .003 |
The implementation of assisted electronic prescribing achieved a reduction in the relative risk of MEs of 96.2% (95% CI, 92.4%-98.1%) a reduction in the absolute risk of MEs of 40% (95%CI, 35.6-44.4%).
CI, confidence interval; IQR, interquartile range; ME, medication error.
When we analysed the source of the error, in the pre-implementation period we found that 125 MEs (59.0%) occurred at the time of prescription and 87 (41.0%) during transcription to the nursing chart. In the post-implementation period, 4 MEs (50.0%) occurred during prescription and 4 (50.0%) during verification of treatment by the pharmacy.
Table 4 summarises the characteristics of the MEs detected in both periods. In the MP period, the most frequent errors were patient identification errors (68/212), followed by errors in the frequency or timing of doses (44/212) and errors in the identification of the prescribed medication (44/212). In the AEP period, we detected a total of 8 errors: 5/8 concerned medication identification (error or confusing information in prescription and/or verification by pharmacist), 2/8 were dosing errors (overdosing) and 1/8 involved selection of a dosage form that did not match the prescribed dose.
Details of the distribution of medication errors in the 2 periods by type of error.
| Type of error | Pre-implementation period (212 errors) n (%) | Post-implementation period (8 errors) n (%) |
|---|---|---|
| Errors in patient identification | 68 (32.1) | 0 (0) |
| Omission of date | 15 (7.1) | 0 (0) |
| Omission of signature | 19 (9) | 0 (0) |
| Illegible signature | 34 (16) | 0 (0) |
| Omission of MRN | 0 (0) | 0 (0) |
| Omission of patient name | 0 (0) | 0 (0) |
| Errors involving the medication | 3 (1.4%) | 0 (0) |
| Errors in frequency/timing of administration | 44 (20.7) | 0 (0) |
| Greater | 6 (2.8) | 0 (0) |
| Lesser | 6 (2.8) | 0 (0) |
| Frequency omitted | 12 (5.7) | 0 (0) |
| Confusing schedule | 20 (9.4) | 0 (0) |
| Errors in dosing | 28 (13.2) | 2 (25) |
| Overdosing | 7 (3.3) | 2 (25) |
| Underdosing | 1 (0.5) | 0 (0) |
| Dose omitted | 6 (2.8) | 0 (0) |
| Dose confusing | 14 (6.6) | 0 (0) |
| Errors in duration of treatment | 3 (1.4) | 0 (0) |
| Longer | 2 (0.9) | 0 (0) |
| Shorter | 1 (0.5) | 0 (0) |
| Error in calculation of dates of administration | 0 (0) | 0 (0) |
| Errors in the route or mode of administration | 21 (9.9) | 0 (0) |
| Incorrect | 3 (1.4) | 0 (0) |
| Illegible | 18 (8.5) | 0 (0) |
| Incorrect dosage form | 0 (0) | 1 (12.5) |
| Duplication | 1 (0.5) | 0 (0) |
| Interactions | 0 (0) | 0 (0) |
| Error in identification of medication | 44 (20.8) | 5 (62.5) |
| Incorrect active ingredient | 8 (3.8) | 0 (0) |
| Incorrect speciality | 3 (1.4) | 0 (0) |
| Medication that could not be substituted | 0 (0) | 0 (0) |
| Transcription omitted | 26 (12.3) | 0 (0) |
| Confusing medication information | 7 (3.3) | 5 (62.5) |
ME, medication error, MRN, medical record number.
The most frequent reason for the error was a lapse/slip (92.9% of MP errors and 50.0% of AEP errors), followed by lack of information about the medication (6.6% of MP errors and 37.5% of AEP errors).
Based on the errors detected in the 2 periods, the implementation of AEP achieved the eradication of the following MEs: errors in patient identification, treatment duration, dosage or route of administration, omission of the dose, and errors related to contraindications in the patient or duplicate prescription. Overall, the introduction of AEP achieved a relative reduction of the risk of MEs of 96.2% (95% CI, 92.4–98.1%) and an absolute reduction of the risk of MEs of 40.0% (95% CI, 35.6–44.4%).
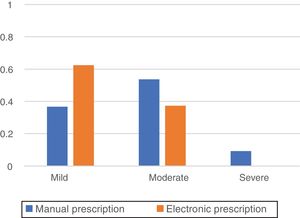
Fig. 1 presents the rating of the detected errors established through the application of the Delphi method in the panel of health professionals. In the pre-implementation period, 9.5% of MEs were considered severe, 53.7% moderate and 36.8% mild, while in the post-implementation period all errors were considered mild (62.5%) or moderate (37.5%).
DiscussionOur study demonstrated that the implementation of AEP in a paediatric setting achieved an absolute risk reduction of MEs of 40% and the eradication of errors considered severe by the care delivery staff. The inclusion of information that can be easily accessed by any member of the health care staff regarding dose adjustments for weight and age, commonly prescribed courses of medication, maximum doses, drug interactions, allergies, consensus protocols and recommendations for the administration and storage of medicines results in improvements in the safety and quality of care delivery. In addition, the elimination of transcription of prescriptions by the nursing staff eliminates a potential source of error.
The classification of MEs varies widely between studies and is probably one of the main reasons for the substantial heterogeneity of the results reported in the literature and the difficulties involved in their comparison. The reduction in MEs following introduction of AEP in the different studies that analysed the paediatric population ranged from 30% to 70%.10,14–16 In our study, we applied the classification developed by NCC MERP adapted by the Ruiz-Jarabo 2000 working group for the standardization of MEs, further modifying it to adapt it to our circumstances by omitting some of the subcategories referring to error reporting, as our sole source of data consisted of the paper-based or electronic health records.12
A common finding in most studies that evaluate the impact of the introduction of AEP is that dosing errors are the type of error avoided most frequently, with a reduction by a percentage that varies from 1% to 15.6%.16–18 We did not observe this reduction, probably due to the few errors detected in the post-implementation period. In our study, patient identification errors were the errors that experienced the greatest reduction (from 32.1% in the MP period to none in the AEP period). The most frequent errors in the MP period were errors related to missing information about the patient or the prescriber in the paper-based prescription order, the dosage or route of administration or illegible prescriptions. Our findings were consistent with those of other studies, with a similar distribution, although other studies have found a greater frequency of dosing errors, errors in the interpretation of the route of administration and omission of a dose interval schedule.19
In our study, we found that the implantation of AEP achieved the eradication of certain errors (patient identification, prescriber identification and signature, prescription date, omission of transcription to nursing chart, illegible or confusing dosage or dose schedule, omission of route of administration, prescription of the wrong active ingredient or wrong speciality), although not all medical errors were eliminated. In fact, we found a total of 8 errors in the post-implementation period. Different studies have observed new errors after the introduction of AEP that did not exist previously, such as duplication of treatment, incorrect selection of medication from a drop-down menu, incorrect selection of the dose due to a typographic error or selection of a predefined regimen that is not appropriate for the patient.17,20 In our study, the verification by the pharmacists of prescriptions ordered by clinicians was key in keeping this type of ME from reaching the patient.
Lapses/slips constituted the main reason for MEs in both the pre-implementation and the post-implementation period. In our study, we did not analyse the potential cause of these lapses, but similar studies have evinced that factors such as burnout, lack of training, stress or fatigue are determinants of the incidence of ME.21 In our study, incorrect use of technology did not emerge as an apparent source of error in the AEP period, contrary to other studies in which this factor was found to account for 19% of errors.20 A possible explanation is that we collected the post-implementation data 3 years after the introduction of AEP, which gave the health care staff time to become familiarised to this technology in everyday clinical practice following several in-person training sessions and the availability of instructions on how to use it in the intranet of the hospital.
Based on the severity rating of MEs by the health care staff, we ought to highlight the absence of errors considered severe in the AEP period and the greater prevalence of errors classified as mild in the AEP period compared to the MP period.
LimitationsThere are limitations to our study. The most important limitation is the absence of a universal scheme for the classification of MEs. Another limitation is that the data for the first period under study (pre-implementation period) were obtained by retrospective review of health records, so that there were errors that we were unable to analyse properly (prescription of an inappropriate/inexistent dosage form). Furthermore, we were unable to determine whether the detected errors in this initial period reached or did not reach the patient. In the post-implementation period, we documented detected errors prospectively, and many were corrected before reaching the patient thanks to verification by the pharmacist.
In order to minimise selection bias, we recruited patients for both periods during the same time of the year to prevent data from not being comparable to data from another time of the year when different diseases would be more prevalent and managed with different medication. Nevertheless, while we carried out the analysis in the same inpatient wards in both periods, we had no way of establishing whether the doctors and nurses involved in medication were the same in the 2 periods.
ConclusionCompared to adults, paediatric patients are at increased risk of experiencing MEs. In addition, these patients are more vulnerable to MEs due to the relative immaturity of their kidneys, liver and immune system. The introduction of e-prescribing with systems to assist the prescription, validation and administration of medication has been proven to significantly reduce MEs and completely eliminate those considered severe by health care staff. The integration of verification by the pharmacist in AEP also allows the identification and correction of MEs before they reach the patient, a process that increases the quality and safety of medication use.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fernández-Oliveira C, Martínez-Roca C, Álvarez-Ávila A, Balboa-Barreiro V, Giménez-Arufe V, Yáñez-Gómez P, et al. Impacto de la implantación de la prescripción electrónica asistida en la seguridad del paciente pediátrico. An Pediatr (Barc). 2020;93:103–110.