Urban air pollution (UAP) is a major threat to child and adolescent health. Children are more vulnerable to its effects, being associated with higher morbidity and mortality due to acute and chronic diseases, especially respiratory ones. A study is performed on the relationship between UAP and the rate of hospital admissions due to acute respiratory diseases.
Patients and methodsAn ecological study was conducted on young people under 19 years-old in the city of Murcia, who had visited hospital emergency departments due to respiratory diseases (ICD-9) during 2015. A logistic regression was performed on the risk of hospital admission that included consultations in relation to the average daily levels of environmental pollutants (NO2, O3, PM10, SO2) obtained from the Air Quality Surveillance and Control network of the Region of Murcia. Other control variables, such as gender, age, average daily ambient temperature, and season of the year.
ResultsA total of 12,354 (56% boys and 44% girls) children consulted in the emergency department for respiratory disease. Of those, 3.5% were admitted, with a mean age of 2.54 (95% CI; 2.16−2.91) years. The Odds Ratio (OR) of hospital admission for respiratory diseases: NO2 1.02 (95% CI; 1.01–1.04; P < .01), O3 1.01 (95% CI; 1.00–1.03; P < .01) male 1.4 (95% CI 1.11–1.79; P < .01) and winter 2.10 (95% CI 1.40–3.21; P < .01). Admissions for asthma: PM10 1.02 (95% CI; 1.01–1.04; P < .05), O3 1.04 (95% CI; 1.01–1.06; P < .01). Admissions for bronchiolitis: Age 0.69 (95% CI; 0.48−0.99; P < .05); NO2 1.03 (95% CI; 1.01–1.05; P < .01).
ConclusionsUAP increases hospital admissions in children due to acute respiratory diseases, especially asthma and bronchiolitis. Implementing preventive measures, expanding time series and collaborative studies with open data, would help improve public health and air quality in the cities.
La contaminación atmosférica urbana (CAU) es una amenaza principal para la salud infanto-juvenil. Los niños son más vulnerables a sus efectos asociando mayor morbimortalidad de enfermedades agudas y crónicas, especialmente respiratorias. Pretendemos estudiar los efectos de la CAU en la tasa de ingresos hospitalarios por causa aguda respiratoria.
Pacientes y métodosEstudio ecológico durante 2015 de las visitas con patología respiratoria (CIE-9) de urgencias hospitalarias en menores de 19 años en el municipio de la ciudad de Murcia. Regresión logística para el riesgo de ingreso hospitalario entre las consultas en relación con los niveles promedios diarios de contaminantes ambientales (NO2, O3, PM10, SO2) obtenidas de la red de Vigilancia y Control de la Calidad del Aire de la Región de Murcia. Otras variables de control: sexo, edad, temperatura ambiental media diaria, y estación del año.
ResultadosUn total de 12,354 niños consultaron en urgencias por patología respiratoria, 56% niños y 44% niñas. Ingresaron el 3,5%, con edad media de 2,54 (IC95% 2,16−2,91) años. Para el riesgo de ingreso hospitalario por enfermedades respiratorias: NO2 1,02 (IC95% 1,01−1,04; P < ,01), O3 1,01 (IC95% 1,00–1,03; P < ,01) sexo masculino 1,4 (IC95% 1,11–1,79; P < ,01) e invierno 2,10 (IC95% 1,40–3,21; P < ,01). Ingresos por asma: PM10 1,02 (IC95% 1,01–1,04; P < ,05), O3 1,04 (IC95% 1,01–1,06; P < ,01) Ingresos por bronquiolitis: edad 0,69 (IC95% 0,48–0,99; P < ,05); NO2 1,03(IC95% 1,01–1,05; P < ,01).
ConclusionesLa CAU aumenta los ingresos hospitalarios en pediatría por patología aguda respiratoria, especialmente por crisis de asma y bronquiolitis. Poner en marcha medidas preventivas, ampliar las series temporales y estudios colaborativos con datos en abierto ayudarían a mejorar la salud pública y la calidad del aire en las ciudades.
Urban air pollution (UAP) is one of the main environmental health threats for the paediatric age group.1 Although the entire population is affected by poor air quality, children and youth are more vulnerable to its effects due to the physiological immaturity of their respiratory and immune systems, their higher metabolism, higher respiratory rate and greater requirements of air per kilogram of body weight compared to adults.2 A growing body of scientific evidence demonstrates the contribution of UAP to the increased morbidity and mortality of children worldwide.3,4 Exposure to UAP during childhood has been associated with an increase in respiratory complaints and associated hospital admissions, inflammation of the airways, impaired respiratory function, asthma attacks and acute lower airway infections.5–15
The World Health Organization (WHO) regularly publishes guidelines on air quality that constitute the main reference to establish standards worldwide.16 It also offers information on the air quality of more than 3000 cities in 103 countries (http://breathelife2030.org/). Table 1 presents the main types of air pollutants highlighted in studies of respiratory morbidity3 and the maximum levels recommended by the WHO.16 The primary components of UAP involved in morbidity are particulate matter, including fine particles 2.5 μm in diameter and smaller (PM2.5) or 10 μm in diameter and smaller (PM10), nitrogen dioxide (NO2), sulphur dioxide (SO2), and ozone (O3). Tropospheric O3 is found in the lowest layers of the atmosphere and is considered a secondary pollutant, as it is not directly emitted to the atmosphere but rather forms from certain precursors (volatile nonmethane organic compounds, carbon monoxide [CO], nitrogen oxides and, to a lesser degree, methane [CH4]) produced by combustion processes (traffic and industry). Through the action of sunlight, these chemical substances react to form O3. Since sunlight is one of the main factors at play in these reactions, the concentrations of these pollutants peak in spring and summer.
Air pollutants that affect the respiratory tract, main sources of these pollutants and mean value limits recommended by the WHO.
| Sources of emissions | WHO limit | |
|---|---|---|
| Particulate matter | Combustion/burning | Annual mean 10 μg/m3 |
| 24-h mean 25 μg/m3 | ||
| Particulate matter <10 μm in diameter (PM10) | Construction, dust resuspended in roads, traffic and wind | Annual mean 20 μg/m3 |
| 24-h mean 50 μg/m3 | ||
| Nitrogen dioxide (NO2) | Emissions from diesel fuels and associated combustion associated with motor vehicle traffic | Annual mean 40 μg/m3 |
| 1-h mean 200 μg/m3 | ||
| Sulphur dioxide (SO2) | Combustion of fossil fuels in industrial plants | 24-h mean 20 μg/m3 |
| 10-min mean 500 μg/m3 | ||
| Ozone (O3) | Photochemical reactions in presence of light and oxides or volatile organic compounds | 8-h mean 100 μg/m3 |
The aim of our study was to analyse the association between the level of UAP and hospital admissions in individuals aged less than 18 years due to acute respiratory illness in the city of Murcia, Spain.
Sample and methodsStudy sampleThe city of Murcia has a population of 441 003 inhabitants (2016), including 100 539 aged less than 19 years.17 The Paediatric Emergency Unit (PED) of the Hospital Clínico Universitario Virgen de la Arrixaca is the only hospital-based paediatric emergency care unit in the city of Murcia in the Murcia public health system.
The population under study consisted of all children aged less than 17 years residing in the city and urban area of Murcia that visited the emergency department of the Hospital Clínico Universitario Virgen de la Arrixaca between January 1, 2015 and December 31, 2015 given any diagnosis of upper or lower respiratory tract infection based on the recorded International Classification of Diseases, Ninth Revision (ICD-9) code. The ICD-9 and hospital admission code were registered by the Department of Clinical Documentation following discharge.
Study variables: hospital admission (yes/no), sex, age in years, grouped into categories (<1 year, 1−3 years, 4−9 years, >9 years), ICD-9 diagnostic code and season of the year.
The study protocol was reviewed and approved by the Clinical Research Ethics Committee of the Hospital Clínico Universitario Virgen de la Arrixaca.
Geographic location and urban air pollution dataMurcia is a town in southeast Spain on the banks of the Segura river in the coastal depression of the Sierra de Carrascoy, 40 km from the Mediterranean, with mild winds and a semiarid dry climate. Its winters are mild and its summers very hot, with an annual mean temperature of 18.6 °C. The area does not have any refineries, thermal power stations or incineration plants based on the Registro Estatal de Emisiones y Fuentes Contaminantes (National Registry of Emissions and Pollution Sources, http://www.prtr-es.es), has experienced considerable urban development and a rapid population increase in the past 25 years. The main sources of pollution are motor vehicle traffic and the burning of biomass or farming residues associated with traditional agriculture in the huerta of Murcia (PM10 and NO2). The periodic intrusion of Saharan dust sometimes causes variations in PM10 levels. The intensity of sunlight and frequent heat in Murcia, combined with UAP and the orography of the area have contributed to an increasing frequency of episodes of excessive levels of the secondary pollutant O3.
We obtained data on environmental pollutants from January 1, 2015 to December 31, 2015 from the website of the Air Quality Surveillance and Control Network of the General Directorate of the Environment of the Region of Murcia.18 It has a measuring station in the square of San Basilio in the city of Murcia. The UAP data obtained for the months under study included the following pollutants: NO2, PM10, O3, SO2. The levels of PM2.5 are not measured. The mean daily temperature was based on the measurements made by the station of the Air Quality Surveillance and Control Network (Murcia-Alcantarilla). We compared these values with the air quality standards of the WHO.
Variables under study: NO2, PM10, O3, SO2 (daily mean), daily mean temperature, month and season of the year of visit.
Statistical analysisWe performed a descriptive analysis with frequency and contingency tables. We fitted a multivariate logistic regression model to assess the risk of admission with a 95% level of confidence. We performed bidirectional tests to prevent confounding due to seasonal variations.19
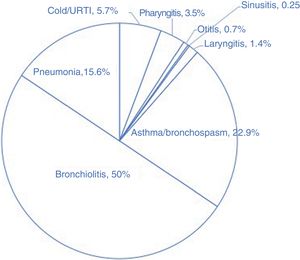
ResultsThe PED received 12 354 visits of residents of the municipality of Murcia due to acute upper or lower respiratory tract disease in 2015. Cold/upper airway infection, asthma/bronchospasm, pharyngitis or sinusitis/abscesses, otitis, laryngitis, bronchiolitis and pneumonia/bronchitis amounted to 38.7%. 18.9%. 14.9%. 10.0%. 7.9%. 6.9% and 2.6, respectively. A total of 436 patients (corresponding to 3.5% of visits for respiratory disease) were admitted to hospital; Fig. 1 shows the distribution of hospital admissions by respiratory disease. Table 2 presents the profiles of the patients that sought care and data on air pollution.
Sociodemographic variables and univariate analysis.
| Discharge home | Admitted to hospital | ANOVA /CW/χ2* | |||
|---|---|---|---|---|---|
| n (%) | Mean (95% CI) | n (%) | Mean (95% CI) | ||
| Sex | .05 | ||||
| Male | 6661 (96.2) | 264 (3.8) | |||
| Female | 5257 (96.8) | 172 (3.2) | |||
| Age (years) | 3.11 (3.06−3.16) | 2.54 (2.16−2.91) | < .01 | ||
| <1 | 2961 (24.0) | 257 (2.1) | |||
| 1−3 | 5726 (46.3) | 91 (0.7) | |||
| 4−9 | 2739 (22.2) | 50 (0.4) | |||
| >/=10 | 492 (4.0) | 38 (0.3) | |||
| Mean T (°C) | 17.35 (17.23−17.47) | 15.26 (14.68−15.84) | < .01 | ||
| Season of year | < .01 | ||||
| Winter | 4850 (40.7) | 255 (58.5) | |||
| Spring | 2509 (21.1) | 76 (17.4) | |||
| Summer | 2375 (19.9) | 49 (11.2) | |||
| Autumn | 2184 (18.3) | 56 (12.8) | |||
| UAP (daily mean µg/m3) | |||||
| NO2 | 46.92 (46.60−47.23) | 53.88 (52.06−55.69) | < .01 | ||
| PM10 | 34.81(34.47−35.16) | 37.13 (35.01−39.25) | .02 | ||
| O3 | 44.35 (43.95−44.75) | 38.27 (36.16−40.38) | < .01 | ||
| SO2 | 7.84 (7.80−7.88) | 7.61 (7.39−7.82) | .02 | ||
CI, confidence interval; UAP, urban air pollution; NO2, nitrogen dioxide; O3, ozone; PM10, particular matter with diameter ≤10 µm; SO2, sulphur dioxide; T, temperature.
Significant variables (P < .05) presented in boldface.
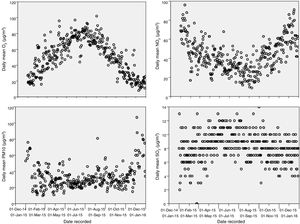
The annual mean level of NO2 in the municipality of Murcia, of 46.92 µg/m3 (95% confidence interval, 46.60−47.23), exceeds the limit recommended by the WHO (40 µg/m3), with levels exceeding this recommended limit 52% of days a year.20 The annual mean of PM10 is 34.81 μg/m3 (34.47−35.16), and the levels of PM10 exceed the annual mean recommended by the WHO (50 µg/m3) 10% of the days. Fig. 2 presents the annual distribution of daily mean values for the 4 pollutants.
Table 3 presents the results of the multivariate logistic regression analysis. The risk of hospital admission due to respiratory complaints was greater in male patients, increased with the mean daily level of NO2 and O3 and decreased with age.
Multivariate logistic regression model. Risk of hospital admission due to acute respiratory illness.
| Risk of hospital admission / all admissions due to respiratory illness | |||||||
|---|---|---|---|---|---|---|---|
| B | SE | Wald | Sig | Exp (B) | Lower bound | Upper bound | |
| Age (years) | –0.035 | 0.021 | 2.661 | 0.103 | 0.966 | 0.926 | 1.007 |
| Sex (male) | 0.346 | 0.121 | 8.260 | 0.004 | 1.414 | 1.116 | 1.791 |
| T (°C) | –0.003 | 0.019 | 0.033 | 0.856 | 0.997 | 0.960 | 1.035 |
| NO2 (µg/m3) | –0.024 | 0.006 | 16.786 | 0.000 | 1.024 | 1.013 | 1.036 |
| O3 (µg/m3) | 0.016 | 0.006 | 6.493 | 0.001 | 1.017 | 1.004 | 1.030 |
| PM10 (µg/m3) | –0.005 | 0.004 | 1.313 | 0.252 | 0.995 | 0.987 | 1.003 |
| SO2 (µg/m3) | –0.021 | 0.028 | 0.602 | 0.438 | 0.979 | 927 | 1.033 |
| Season of year | 15.421 | 0.001 | |||||
| Winter | 0.744 | 0.215 | 11.993 | 0.001 | 2.105 | 1.403 | 3.211 |
| Spring | 0.130 | 0.246 | 0.279 | 0.597 | 1.139 | 0.879 | 1.846 |
| Summer | –0.158 | 0.296 | 0.284 | 0.594 | 0.854 | 0.478 | 1.525 |
| Constant | –5.285 | 0.583 | 82.133 | 0.000 | 0.005 | ||
| Risk of hospital admission / asthma | |||||||
|---|---|---|---|---|---|---|---|
| B | SE | Wald | Sig | Exp (B) | Lower bound | Upper bound | |
| Age (years) | 0.040 | 0.036 | 1.221 | 0.269 | 1.041 | 0.969 | 1.118 |
| Sex (male) | 0.481 | 0.268 | 3.217 | 0.073 | 1.618 | 0.956 | 2.738 |
| T (°C) | –0.028 | 0.040 | 0.495 | 0.482 | 0.972 | 0.898 | 1.052 |
| NO2 (µg/m3) | 0.014 | 0.012 | 1.356 | 0.244 | 1.014 | 0.990 | 1.039 |
| O3 (µg/m3) | 0.036 | 0.013 | 7.835 | 0.005 | 1.037 | 1.011 | 1.063 |
| PM10 (µg/m3) | 0.019 | 0.008 | 5.194 | 0.023 | 1.019 | 1.003 | 1.036 |
| SO2 (µg/m3) | 0.005 | 0.062 | 0.008 | 0.931 | 1.005 | 0.891 | 1.134 |
| Season of year | 2.691 | 0.442 | |||||
| Winter | –0.017 | 0.448 | 0.001 | 0.970 | 0.983 | 0.409 | 2.365 |
| Spring | –0.710 | 0.502 | 1.998 | 0.158 | 0.492 | 0.184 | 1.316 |
| Summer | –0.424 | 0.544 | 0.609 | 0.435 | 0.654 | 0.225 | 1.899 |
| Constant | –5.970 | 1.195 | 24.958 | 0.000 | 0.003 | ||
| Risk of hospital admission / bronchiolitis | |||||||
|---|---|---|---|---|---|---|---|
| B | SE | Wald | Sig | Exp (B) | Lower bound | Upper bound | |
| Age (years) | –0.365 | 0.184 | 3.927 | 0.048 | 0.694 | 0.484 | 0.996 |
| Sex (male) | 0.377 | 0.206 | 3.354 | 0.067 | 1.457 | 0.974 | 2.180 |
| T (°C) | –0.006 | 0.035 | 0.034 | 0.854 | 0.994 | 0.928 | 1.064 |
| NO2 (µg/m3) | 0.031 | 0.010 | 10.031 | 0.002 | 1.032 | 1.012 | 1.052 |
| O3 (µg/m3) | 0.019 | 0.011 | 2.953 | 0.086 | 1.019 | 0.997 | 1.041 |
| PM10 (µg/m3) | –0.008 | 0.007 | 1.497 | 0.221 | 0.992 | 0.979 | 1.005 |
| SO2 (µg/m3) | 0.001 | 0.046 | 0.001 | 0.979 | 1.001 | 0.915 | 1.096 |
| Season of year | 4.254 | 0.235 | |||||
| Winter | 0.342 | 0.375 | 0.832 | 0.362 | 1.408 | 0.675 | 2.938 |
| Spring | 0.547 | 0.417 | 1.721 | 0.190 | 1.729 | 0.763 | 3.916 |
| Summer | –0.920 | 0.884 | 1.084 | 0.298 | 0.398 | 0.070 | 2.252 |
| Constant | –3.388 | 1.114 | 9.252 | 0.002 | 0.034 | ||
Input variable(s): NO2, nitrogen dioxide; O3, ozone; PM10, particulate matter ≤10 µm; SO2, sulphur dioxide; daily mean, season of year, daily mean temperature in the city of Murcia, age in years, sex.
Our findings reveal that a higher short-term level of UAP (O3 and NO2), male sex and winter were associated with an increased risk of hospital admission due to respiratory illness. This association was strongest for asthma (with O3 and PM10) and bronchiolitis (with NO2).
The increased hospital admission rate in male patients has already been described in the previous literature,14,15 although it diverges from the increased vulnerability to O3 described in female patients.21 Some of the possible explanations proposed for this are models of exposure (boys tend to play outdoors for longer periods and engage in more aerobic activity) and physiological (differences in hormones, lung size, inflammation of airways).
From a physiological standpoint, it makes sense that air pollutants could increase the severity of asthma and bronchiolitis on account of their known effect on lung function and airway inflammation.22
There is evidence that short-term exposure to particulate matter, O3,18 SO2 and NO2 is associated to an increase in asthma exacerbations.10 The effect of PM10 on asthma could be explained by the oxidative stress induced by the presence of PM10 in the epithelium, with an increase in prostaglandin E that leads to defective macrophage phagocytosis, inflammation, oedema and cytotoxicity. The evidence on hospital admissions due to asthma in relation to O3 exposure requires further research,14,23,24 but a review of 87 studies found that 71 reported a significant increase in the risk of hospital admission due to asthma.15
Bronchiolitis is an infection of the small airways that mainly affects children aged less than 2 years. It causes inflammation of the airways and necrosis of the bronchial epithelium.25 In 80% of cases it is associated with infection by respiratory syncytial virus, and it is one of the main reasons for hospital admission in children aged less than 2 years in both high-income26 and low-income27 countries. Although there are isolated cases throughout the year, there are usually one to two outbreaks of bronchiolitis in the cold months of the year, between November and March. The risk factors known to increase the risk of hospital admission are preterm birth, low birth weight, heart disease or exposure to tobacco smoke. We found an association between the mean daily level of NO2 and the risk of hospital admission due to bronchiolitis. The current evidence suggests an association between short (<24 h) and recent exposure to PM2.5; PM10; NO2 and SO2, among other pollutants, and an increased incidence of lower respiratory tract infections11 and an increased risk of hospital admission due to bronchiolitis.28 The greater effect observed in other studies with chronic exposure to particulate matter (several days or weekly mean) on hospital admission due to bronchiolitis may reflect different pathogenic mechanisms.29 Chronic exposure to PM2.5 and PM10 may have a chronic proinflammatory effect,30 whereas exposure to NO2 and SO2 may be associated with more acute airway damage.31
Ozone levels usually peak during hot seasons, which are far apart from the periods when outbreaks of infection by respiratory syncytial virus tend to occur. This would make it difficult to identify an association with bronchiolitis. In winter, other contaminants, such as NO2, are probably more relevant contributors to respiratory disease. As has been done by other authors,32 we analysed synergistic effects and interactions in a model taking into account multiple pollutants and age groups, and we did not find any differences in the results.
In our study, the annual mean level of NO2 in the city of Murcia exceeded the recommended limit for the health of its residents. Murcia is the city that ranks 8th in Spain in terms of the highest annual mean level of NO2. The increasing urbanization and concentration of the population in megacities,33 in absence of corresponding air quality control measures, has a direct impact on child health. At present, 98% of cities in low- and medium-income countries and 56% of cities in high-income countries have levels of pollution exceeding the limits recommended by the WHO.34
One significant limitation of our study involves the scarcity of sources of pollution data. The municipality of Murcia has 400 000 inhabitants, making it the seventh most populous municipality in Spain, yet it only has one pollutant measurement station, which does not collect information for PM2.5, among other pollutants. The season and the temperature play a role in the variations in levels of UAP, so we controlled our analyses for these factors.35–39 Other population- and individual-based variables, such as smoking, dietary factors and lifestyle, may be a source of bias. Smoking is particularly important, given that approximately 50% of children in Spain live with a smoker at home.40 The low level of documentation and the lack of an established coding scheme to document exposure to second-hand smoke in minors that visited the PED precluded the analysis of its impact on our findings. We believe it essential to train paediatricians in the use of brief opportunistic intervention during PED visits. On the other hand, additional collaborative cohort and longitudinal studies with a standardised methodological approach are needed to better analyse the associations identified in our study.
Although the proportion of patients requiring hospital admission due asthma or bronchiolitis that could be attributed to UAP may seem small, UAP probably has a substantial impact in morbidity and the global burden of disease at the population level. The good news is that exposure to UAP is totally preventable. Successful interventions are already being developed and implemented in numerous cities and regions across the globe. The reduction of UAP has been associated with public health benefits, with a decrease in mortality associated to cardiovascular and respiratory diseases in adults and a decrease in the rate of hospital admission due to respiratory complaints in children (of up to 87% in cities that started out with high levels of pollutants), a decrease in the incidence of preterm birth and a reduction in lung inflammation.33,41–45 Thus, improving air quality surveillance systems and implementing measures for control of UAP (public transport, urban design, banning brush burning, promoting cycling, limiting the use of high-emission motor vehicles, improving monitoring of UAP and its effects in vulnerable groups, among others) contribute to reducing the rate of hospital admission in the paediatric age group.
Such a pathway requires courageous leadership, the creation of new structures that connect health and environment, substantial resources and widespread changes in society.40 The growing social awareness of the association between health and environment is a key driver of change. We take this opportunity to ask Spanish paediatricians to help increase the sample size, providing free access to our data (http://pehsu.org/wp/wp-content/uploads/UAP_IngresosRespi.zip) to other paediatrics research groups in Spain. The combined analysis of clinical and environmental surveillance data is a powerful tool toward achieving adequate monitoring and for creating models that can contribute to improving health and air quality in cities.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Dr Picazo Román and his entire clinical documentation team at the Hospital Clínico Universitario Virgen de la Arrixaca for their collaboration in the project. We thank the groups Madres por el Clima Murcia (Murcia Mothers for Climate) and Stop Quemas Murcia (Stop Burning Murcia) for their work in raising social awareness of the need to improve air quality in the city of Murcia.
Please cite this article as: Ortega-García JA, Martínez-Hernández I, Boldo E, Cárceles-Álvarez A, Solano-Navarro C, Ramis R, et al. Contaminación atmosférica urbana e ingresos hospitalarios por asma y enfermedades respiratorias agudas en la ciudad de Murcia (España). An Pediatr (Barc). 2020;93:95–102.