Acute lymphoblastic leukaemia (ALL) has been associated with an excess of minor phenotypic variants (MPV), including common variants and minor anomalies, indicative of an altered phenogenesis. The objective of the study was to determine the association between MPV and ALL.
Patients and methodsIn a hospital based case–control study, we studied 120 children with ALL (including standard and high risk) and 120 healthy children as a control group, matched for age and sex, seen in the Hospital Civil de Guadalajara Dr. Juan I. Menchaca (Guadalajara, Mexico). In both groups, 28 anthropometric measurements were made, as well as a systematic search for 405 MPV, through a physical examination. Adjusted odds ratio was estimated (aOR) with its intervening variables by logistic regression. The confidence interval was 95% (95%CI).
ResultsAnthropometric signs associated with ALL were: long upper segment (aOR=2.19, 95%CI: 1.01–4.76), broad jaw (aOR=2.62, 95%CI: 1.29–5.30), narrow ears (aOR=6.22, 95%CI: 2.60–14.85), and increase in internipple distance (aOR=2.53, 95%CI: 1.07–5.98). The hypoplasia mesofacial, broad forehead, small nose, short columella, narrow ears, telethelia, Sydney crease (SC), Greek type feet and café-au-lait spots (CALS), had a 3–17 times higher frequency in children with ALL. By number, an association was found from ≥4 MPV (aOR=2.14, 95%CI: 1.25–3.66, P=.004).
ConclusionsFrom ≥4 MPV, an association was found with ALL, suggesting prenatal factors in phenogenesis and leukemogenesis. CALS and SC were confirmed as MPV in children with ALL.
La leucemia linfoblástica aguda (LLA) se ha asociado a un exceso de variantes fenotípicas menores (VFM), que incluyen las variantes comunes y las anomalías menores, indicadoras de una fenogénesis alterada. El objetivo fue determinar la asociación entre VFM y LLA.
Pacientes y métodosEstudio de casos y controles basado en hospital de 120 niños con LLA y 120 niños sanos como grupo control, emparejados por edad y sexo, atendidos en el Hospital Civil de Guadalajara Dr. Juan I. Menchaca (México). En ambos grupos, se realizaron 28 mediciones antropométricas y la búsqueda sistemática de un listado de 405 VFM mediante un examen físico minucioso. Se estimaron las odds ratio ajustadas (ORa) con sus variables intervinientes por regresión logística. El intervalo de confianza fue del 95% (IC del 95%).
ResultadosLos signos antropométricos asociados con LLA fueron: segmento superior largo (ORa=2,19; IC del 95%, 1,01–4,76), mandíbula ancha (ORa=2,62; IC del 95%, 1,29–5,30), pabellones estrechos (ORa=6,22, IC95%: 2,60–14,85) y teletelia (ORa=2,53; IC del 95%m 1,07–5,98). Las VFM hipoplasia mesofacial, frente ancha, nariz pequeña, columnela corta, pabellones estrechos, teletelia, línea Sídney, pie griego y manchas café con leche (MCL) tuvieron una frecuencia de 3 a 17 veces mayor en los niños con LLA. Por número, encontramos asociación a partir de ≥4 VFM (ORa=2,14; IC del 95%, 1,25–3,66; p=0,004).
ConclusionesA partir de ≥4 VFM, se encontró asociación con LLA, lo que indica la existencia de factores prenatales en la fenogénesis y leucemogénesis. Confirmamos las MCL y la línea Sídney como VFM asociada a niños con LLA.
According to the comprehensive proposal of Merks et al., morphological abnormalities can be classified as: (a) major anomalies, which include malformations (defects in embryogenesis) and other non-malformational abnormalities, such as severe deformations, disruptions and dysplasias, and (b) minor phenotypic variants (MPVs) or defects of phenogenesis, subdivided, according to their prevalence in the general population, into minor anomalies (MA) (≤4%) and common variants (CV) (>4%). Different types of morphological abnormalities have been associated with various types of childhood cancer, although these studies have focused mainly on major anomalies, both in isolation in syndromes (e.g., aniridia and Wilms tumour in WAGR complex) and non-syndromic (e.g., congenital cardiopathies and malformations of the pancreatic–digestive tract with leukemias, or malformations of the central nervous system and cerebral tumours).2–5 Moreover, it is well known that there is a predisposition to cancer in aneuploidies (acute lymphoblastic leukaemia [ALL] with Down syndrome),6 microdeletion/duplication syndromes (Wilms tumour and other embryonal tumours in Wiedemann–Beckwith syndrome)7 and several monogenic disorders with chromosomal instability (leukaemia in Fanconi anaemia).8 Studies on major anomalies in children with cancer2–4,6–15 have identified this clear association and have contributed significantly to knowledge of the participating genes, both in carcinogenesis and in organogenesis, although the aetiology of many of these associations remains elusive, and these studies have the disadvantage of having been carried out on the basis of individual medical files, population registers or telephone interviews.
Equally important, though less studied, MPVs are easily recognisable physical characteristics that have a minimal effect on a child's normal development and that can serve as indicators of abnormal phenogenesis and can therefore also indicate the prenatal origin of a particular cancer.16–24 Unlike major anomalies, the study of MPVs requires a thorough external physical examination and anthropometric measurements for each patient, but they have the disadvantage that many MPVs are assessed subjectively, and varying terminology and classifications are used to refer to them.25,26 Association studies between MPVs and various types of solid tumours in children18,23 and haemotologic neoplasms16,19–21,27,28 agree that these children have an excessive number of MPVs, but no consensus has yet been reached as to the specific types or patterns of associated MPVs. Acute leukemias are the main type of neoplasm in children and in Mexico it is estimated that between 1000 and 2000 new cases of this cancer are diagnosed every year,29 the highest percentage of which corresponds to ALL. The objective of this study was to make a clinical and anthropometric assessment of the reported association between the presence of MPVs (including MAs and CVs) and ALL, in patients receiving care in a highly specialised unit, authorised to provide treatment in Haemotology for patients in Seguro Popular, the Mexican Government's national health insurance scheme.
Patients and methodsSample profileWith a hospital-based case and control design, we studied children diagnosed with ALL (including both standard- and high-risk) being treated at the Leukaemia Clinic of the Paediatric Haemotology and Oncology Service at the Hospital Civil de Guadalajara Dr. Juan I. Menchaca (Guadalajara, Mexico) during the period 2009–2012. The study sample included 120 children with non-syndromic ALL, that is, ALL not affected by any genetic entity, as in the cases. The type of leukaemia was confirmed in all cases on the basis of morphology in bone marrow smear, flow cytometry and cytogenetic analysis, following the World Health Organization's diagnosis and classification criteria (2008).30 The oncological data were obtained from the hospital file and the rest of the patient's history from questioning of the mother by one of the investigators who was trained for this purpose. Enrolled in the control group were 120 children considered healthy by their mothers, with no apparent dysmorphias or developmental problems and whose personal and family history was negative for cancer, selected by a simple random process among brothers and sisters accompanying other children who attended the paediatric outpatient department of our hospital with some acute infectious condition, and were the same sex and age as the cases.
Anthropometric assessmentFor each of the study subjects, 28 different anthropometric measurements were taken to assess their morphotype, skull, face, torso and limbs, including weight (W), height (H), upper segment (US), lower segment (LS), head circumference (HC), anteroposterior, biparietal and bizygomatic head diameter, upper, mid, lower and total facial length, mandibular width, inner and outer canthal distance, palpebral fissures, ear width, columella length, interalar distance, philtrum length, intercommissural distance, total length of upper limbs, hand length, total length of lower limbs, foot length, chest circumference, internipple distance and sternal length. All the measurements were taken by the same person (S.A.E.P.), following training and standardisation. The appropriate instruments were used for each measurement: infantometer, wall stadiometer, paediatric and standing scales, non-stretchable tape measure, anthropometer and Vernier calliper. In the measuring techniques and reference values for W, H, US, LS and HC we followed Ramos-Galván,31 and for the rest we used those included in the anthropometric handbooks by Hall et al.32 and Lapunzina and Aiello,33 interpreting each of them according to the normal limits for each measurement by age and sex.
Assessment of minor morphological variantsIn the presence of one of the parents, we conducted a thorough, systematic physical examination of each case and his or her control, aimed at detecting MPVs. We used a modified version of the list of MPVs proposed by Merks et al.1 to record the presence or absence of 405 physical variants (132 CVs and 273 MAs), grouped into 29 body regions. The definitions used for each of the MPVs studied were taken from conventional dysmorphological references.34,35 All the MAs and VCs studied met the requirement of being able to be fully assessed by external examination, which was carried out by doctors with experience in clinical dysmorphology. Major internal anomalies were not included, as these require supplementary assessments in addition to the physical examination.
Statistical analysisThe averages of the anthropometric measurements were compared using the Student's t-test. The frequencies of the demographic features and of the phenotypic abnormalities were compared using the chi-squared test. The association between the type and number of MPVs and ALL was measured by means of the odds ratio (OR). The effect of the variables involved in the association between type and number of MPVs and ALL was assessed by logistic regression. In all the statistical calculations the confidence interval chosen was 95% (95%CI).
The investigation was approved by the Ethics and Research Committees of our hospital and informed consent to participate in the study was obtained for all the study subjects.
ResultsOf the 120 patients with ALL, 71 were male (59.2%) and 49 female (40.8%), and the control group contained equal numbers of participants. All were of mixed race. Among the socio-demographic characteristics and histories of the study subjects, the notable points were that the children with ALL had a higher birth order and their mothers were of higher average age with a higher number of gestations and a lower frequency of smoking, whilst the fathers of children with ALL showed a higher frequency of low educational level. The remaining socio-demographic characteristics and histories of the study subjects were similarly distributed in the two study groups (Table 1).
Socio-demographic characteristics and histories of the study subjects.
| Variables | Children with ALL n=120, mean±SD | Control group n=120, mean±SD | Pa |
|---|---|---|---|
| Age of index case (years) | 6.5±3.4 | 6.0±3.3 | 0.200 |
| Father's age (years) | 35.8±7.9 | 34.5±6.1 | 0.168 |
| Mother's age (years) | 33.2±7.8 | 31.3±6.8 | 0.045 |
| Number of gestations | 3.5±1.9 | 2.7±1.0 | 0.001 |
| Gestation order of index case | 2.5±1.7 | 1.9±1.1 | 0.001 |
| Gestational age (weeks)b | 39.0±2.0 | 38.8±1.9 | 0.648 |
| Birth weight (g) | 3262.9±457.9 | 3238.4±496.7 | 0.705 |
| n (%) | n (%) | ||
|---|---|---|---|
| Birth weight (g) | |||
| <2500 | 6/105 (5.7) | 5/113 (4.4) | 0.449 |
| 2500–2999 | 22/105 (20.9) | 23/113 (20.3) | 0.523 |
| 3000–3999 | 71/105 (67.6) | 81/113 (71.7) | 0.307 |
| ≥4000 | 6/105 (5.7) | 4/113 (3.5) | 0.329 |
| Mother's education | |||
| ≤7 years | 53/120 (44.2) | 40/120 (33.3) | 0.118 |
| 7–12 years | 43/120 (35.8) | 42/120 (35.0) | 1.000 |
| ≥12 years | 24/120 (20.0) | 38/120 (31.7) | 0.055 |
| Mother with chronic illness | 13/120 (10.8) | 9/120 (7.5) | 0.502 |
| Mother consumes alcohol | 16/120 (13.3) | 11/120 (9.2) | 0.413 |
| Mother smokes | 7/120 (5.8) | 18/120 (15.0) | 0.035 |
| Father's education | |||
| <7 years | 60/120 (50.0) | 39/115 (33.9) | 0.018 |
| 7–12 years | 41/120 (34.2) | 45/115 (39.1) | 0.513 |
| ≥12 years | 19/120 (15.8) | 31/115 (26.9) | 0.054 |
| Father with chronic illness | 13/120 (10.8) | 4/117 (3.4) | 0.050 |
| Father consumes alcohol | 59/119 (49.6) | 49/117 (41.8) | 0.290 |
| Father smokes | 15/119 (12.6) | 21/117 (17.9) | 0.336 |
| Bloodrelationship | 6/120 (5.0) | 1/120 (0.8) | 0.059 |
SD, standard deviation; ALL, acute lymphoblastic leukaemia.
Of the 28 anthropometric measurements taken in both groups, only nine showed significant statistical differences in the children with ALL (Table 2). Excluding the measurements in which an extreme variation was identified, these nine measurements were subsequently translated into nine clinical signs on the basis of their anthropometric interpretation, and it was observed that three showed an association with ALL in the bivariate analysis (Table 3). A multivariate model was constructed using logistic regression with the forced entry method, which included age, sex and birth weight as intervening variables and showed that their adjusted ORs continued to be significant only for long US, broad jaw, narrow ears and wide-spaced nipples (Table 3).
Anthropometric measurements in children with leukaemia and their control group.
| Measurement | Children with ALL n=120, mean±SD | Control Group n=120, mean±SD | Pa |
|---|---|---|---|
| Weight (kg) | 25.91±12.43 | 22.65±11.24 | 0.036 |
| Height (cm) | 117.71±22.23 | 114.08±18.89 | 0.175 |
| Upper segment (cm) | 62.39±8.58 | 58.92±9.11 | 0.003 |
| Lower segment (cm) | 56.45±11.96 | 55.9±13.13 | 0.735 |
| Head circumference (cm) | 50.50±2.16 | 50.67±2.26 | 0.561 |
| Anteroposterior head diameter (cm) | 17.17±0.73 | 17.15±1.16 | 0.883 |
| Biparietal head diameter (cm) | 13.74±0.89 | 13.81±0.76 | 0.533 |
| Bizygomatic diameter (cm) | 10.94±0.91 | 10.79±1.01 | 0.238 |
| Upper facial length (cm) | 9.69±1.50 | 9.63±1.31 | 0.761 |
| Midfacial length (cm) | 4.49±0.67 | 4.37±0.68 | 0.328 |
| Lower facial length (cm) | 5.57±0.58 | 5.53±0.72 | 0.763 |
| Total length of face (cm) | 9.92±0.92 | 9.84±0.95 | 0.524 |
| Mandibular width (cm) | 9.27±0.87 | 8.83±0.85 | <0.0001 |
| Inner canthal distance (cm) | 2.97±0.26 | 2.85±0.30 | 0.001 |
| Outer canthal distance (cm) | 8.14±0.93 | 8.00±0.58 | 0.199 |
| Palpebral fissures (mm) | 26.40±1.93 | 25.90±2.24 | 0.094 |
| Ear width (mm) | 30.76±2.37 | 32.63±3.84 | <0.0001 |
| Columella length (mm) | 6.09±1.16 | 6.33±1.29 | 0.136 |
| Interalar distance (mm) | 28.98±2.63 | 28.35±2.84 | 0.081 |
| Philtrum length (mm) | 10.90±2.59 | 11.30±2.29 | 0.215 |
| Intercommissural distance (cm) | 3.74±0.49 | 3.53±0.51 | 0.005 |
| Total length of upper limb (cm) | 50.88±10.99 | 49.17±10.60 | 0.220 |
| Hand length (cm) | 13.79±2.11 | 13.23±2.05 | 0.040 |
| Total length of lower limb (cm) | 59.63±12.34 | 57.69±13.85 | 0.220 |
| Foot length (cm) | 18.73±2.99 | 17.69±3.01 | 0.008 |
| Chest circumference (cm) | 60.36±7.88 | 57.17±8.05 | 0.257 |
| Internipple distance (cm) | 14.81±2.73 | 13.77±2.13 | 0.001 |
| Sternal length (cm) | 12.00±2.13 | 11.55±1.51 | 0.071 |
SD, standard deviation; ALL, acute lymphoblastic leukaemia.
Anthropometric signs associated with children with acute lymphoblastic leukaemia.
| Signs | Children with ALL, n=120a, % | Control group, n=120a, % | Pb | Odds ratio | |
|---|---|---|---|---|---|
| Raw | Adjustedc | ||||
| Overweight | 13.5 | 9.8 | 0.250 | 1.44 (0.63–3.25) | 0.79 (0.33–1.88) |
| Long upper segment | 25.5 | 14.7 | 0.039 | 1.98 (0.98–3.99) | 2.19 (1.01–4.76) |
| Broad jaw | 31.3 | 17.7 | 0.018 | 2.11 (1.09–4.05) | 2.62 (1.29–5.30) |
| Telecanthus | 3.6 | 2.5 | 0.462 | 1.44 (0.31–6.60) | 1.53 (0.30–7.70) |
| Narrow ears | 37.6 | 7.5 | <0.0001 | 7.47 (3.21–19.36) | 6.22 (2.60–14.85) |
| Macrostomia | 2.5 | 1.0 | 0.419 | 2.51 (0.22–28.30) | 2.93 (0.25–33.28) |
| Distal elongation of hands | 10.0 | 16.9 | 0.086 | 0.54 (0.25–1.17) | 0.66 (0.29–1.50) |
| Distal elongation of feet | 7.6 | 7.9 | 0.566 | 0.96 (0.36–2.52) | 1.09 (0.41–2.89) |
| Wide-spaced nipples | 18.6 | 8.2 | 0.018 | 2.52 (1.11–5.87) | 2.53 (1.07–5.98) |
ALL, acute lymphoblastic leukaemia.
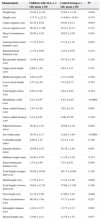
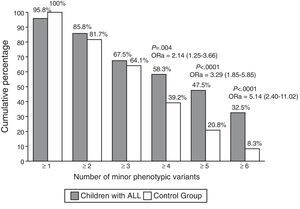
The total number of MPVs found in the group of children with ALL (including CVs and MAs) was 629 (mean±standard deviation of 5.6±3.3 per patient) compared with 497 (mean of 4.2±1.7 per child) in the control group (P<0.0001). A comparison of the cumulative percentages of children in the two groups with one or more and up to 6 or more MPVs (Fig. 1) showed significant differences only from four or more MPVs in the children with ALL, and their ORs adjusted for age and sex were two to five times higher than in the control group.
Cumulative percentage of minor phenotypic variants (MPVs). We present the odds ratios of the percentages of MPVs that appeared 2–7 times more often in children with ALL compared with the children in the control group; the P-value in all these cases was less than 0.05. aOR, adjusted odds ratio (95% confidence interval).
In the bivariate analysis there were nine MPVs observed in the physical examination that occurred 3–17 times more often in the children with ALL than in the controls and whose multivariate analysis showed only marginal changes in the strength of association (Table 4). By MPV type, midfacial hypoplasia, narrow ears and wide-spaced nipples were MAs and the rest were CVs. Although an association was also found between ALL and the MPVs sparse hair, dry hair, full cheeks and dry skin, we have not included them in Table 4, as we attribute them to the treatment received or to the illness itself. For the remaining frequencies of the MPVs assessed we did not find any association with the presence of ALL.
Minor phenotypic variants associated with children with acute lymphoblastic leukaemia.
| Type of abnormality | Children with ALL, n=120, % | Control group, n=120, % | Pa | Odds ratio | |
|---|---|---|---|---|---|
| Raw | Adjustedb | ||||
| Midfacial hypoplasia | 10.0 | 0 | 0.0002 | 14.43 (2.09–618.24) | 14.26 (1.81–111.87) |
| Broad forehead | 8.4 | 0 | 0.007 | 11.99 (1.68–520.29) | 11.77 (1.47–93.93) |
| Small nose | 18.3 | 4.1 | 0.001 | 5.16 (1.81–18.0) | 4.95 (1.78–13.79) |
| Short columella | 18.3 | 5.8 | 0.005 | 3.62 (1.41–10.43) | 3.50 (1.41–8.67) |
| Narrow ears | 12.5 | 0 | 0.0004 | 18.26 (2.73–772.08) | 17.06 (2.19–132.49) |
| Wide-spaced nipples | 6.6 | 0.8 | 0.017 | 8.50 (1.10–380.26) | 9.37 (1.14–76.72) |
| Sydney crease | 14.1 | 1.6 | 0.0008 | 9.74 (2.21–88.25) | 9.19 (2.04–41.41) |
| Morton's toe | 9.2 | 1.6 | 0.0225 | 5.95 (1.25–56.13) | 5.82 (1.24–27.32) |
| Café-au-lait spots | 10.8 | 0.8 | 0.0024 | 14.46 (2.09–619.47) | 14.26 (1.81–111.87) |
ALL, acute lymphoblastic leukaemia.
With reference to clinical association studies using MPVs, Méhes et al.27 stress the importance of conducting such studies with the strictest possible objectivity and recommend using a list to remind the examiner of the presence or absence of each MPV and, ideally, its anthropometric assessment when looking for correspondences with the physical examination, bearing in mind the possible influence of factors such as ethnic group, sex, age or familial variations, and also taking care not to include subjects with recognisable syndromes. Taking account of almost all these elements, our results support the hypothesis relating to the existence of an aetiologic or pathogenic common factor between foetal development and leukemogenesis,16–23 although these results are limited in terms of the specific type of MPV. Other risk factors reported for ALL,36 such as the mother's age, exposures during pregnancy and birth weight of children who develop ALL, together with strong genetic evidence, such as disruption of the mixed lineage leukaemia gene (MLL) in children less than a year old with ALL,37 also support this initiation in utero. Regarding these factors, the mothers of children with ALL in our study were older and had had a larger number of gestations, and therefore the children with ALL had a higher birth order among their respective siblings (Table 1). Von Behren et al.38 attribute this risk to the fact that the ova are exposed for longer to unknown potential environmental agents.
Given that ALL is the most common childhood cancer,29 we consider that it is worth studying separately in relation to MPVs. The differences between the previous studies18–21,27 in terms of the sample size (from 49 to 195 patients with ALL), number of MPVs studied (from 55 to 405) and their type (MAs only or CVs and MAs) make it difficult to compare the results. The use of a list which included almost all known CVs and MAs in humans1 explains the fact that we found a higher number of MPVs. Despite these limitations, the previous studies18–21,27 and our own agree in finding that children with leukaemia have an excess of MPVs, which indicates that one or more prenatal factors cause both MPVs and cancer, although they have not enabled us to identify the associated types of MPVs conclusively. The MPVs that seem to emerge by showing an association or statistically significant differences in children with leukaemia in at least 2 or more studies include variations in palmar flexion creases,18,21 pigmented naevi19,20 and café-au-lait spots (CALS),19,20,27 whilst hypertelorism, epicanthus, synophrys, high arched palate, low-set ears, absent ear lobule, syndactyly, clinodactyly and hallux valgus are MPVs reported in a single study.21 A Sydney crease is a variation in the palmar flexion creases originally described in children with leukaemia by Menser and Purvis-Smith, and is an age-dependent feature,39 defined as an extension of the five-finger crease to the ulnar border of the palm. Our results, together with those of Méhes et al.18 and Durmaz et al.,21 allow us to identify it as a morphological characteristic associated with children with leukaemia. The reported association with CALS,19,20,27,28 also observed by us, has been confirmed in the absence of neurofibromatosis or other entities associated with CALS.7,8 The association between CALS and leukaemia is interesting, as it also illustrates the phenotypic overlap that exists between patients with multifactorial cancer and monogenic cancer syndromes, a fact that may contribute to the future identification of causal genes.2,7,8 By means of progressive statistical analysis Merks et al.22 identified two patterns of morphological abnormalities in children with cancer: (a) the blepharophimosis pattern (blepharophimosis together with increased anterior-posterior angulation of the spine, patchy hypopigmentation of the skin and multiple CALS), and (b) the asymmetric lower limb pattern (asymmetric lower limbs together with tall stature, midface hypoplasia or hypoplastic malae, ptosis and pectus excavatum or carinatum). In this connection, we again highlight CALS and midface hypoplasia as findings also observed in our study. We also find that few studies include an anthropometric assessment for defining the presence of the various MPVs. Méhes et al.18 report a significantly higher frequency of microcephaly in children with leukaemia; however, this finding was not confirmed in our study, and neither was the tall stature reported by Merks et al.22 Although they were not observed in the previous studies,16,18 we highlight the presence of narrow ears and wide-spaced nipples observed in the physical examination and confirmed anthopometrically in our study (Tables 3 and 4), since they may constitute reproducible elements for consideration as further candidate signs associated with ALL.
As for their significance, Méhes and Kosztolányi40 consider that phenotypic abnormalities constitute a clinical manifestation of the genomic instability which causes cancer, although they must be regarded as non-specific associations. The concept of genomic instability is also related to the familial incidence of cancer and its accompanying disorders, whether immunological, non-immunological, of growth, development, reproduction or premature ageing, since these are inherent to, or associated or overlapping with, the development of malign neoplasms.18,27,40 In conclusion, the findings of our study and those previously published in the literature indicate that the association of specific patterns of MPVs in children with leukaemia is of a provisional nature, and further studies are needed in the future, using the new genetic technologies, to distinguish those variations that have real pathogenic or aetiologic significance.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Estrada-Padilla SA, Corona-Rivera JR, Sánchez-Zubieta F, Bobadilla-Morales L, Corona-Rivera A. Variantes fenotípicas menores en pacientes con leucemia linfoblástica aguda del occidente de México. An Pediatr (Barc). 2015;82:75–82.