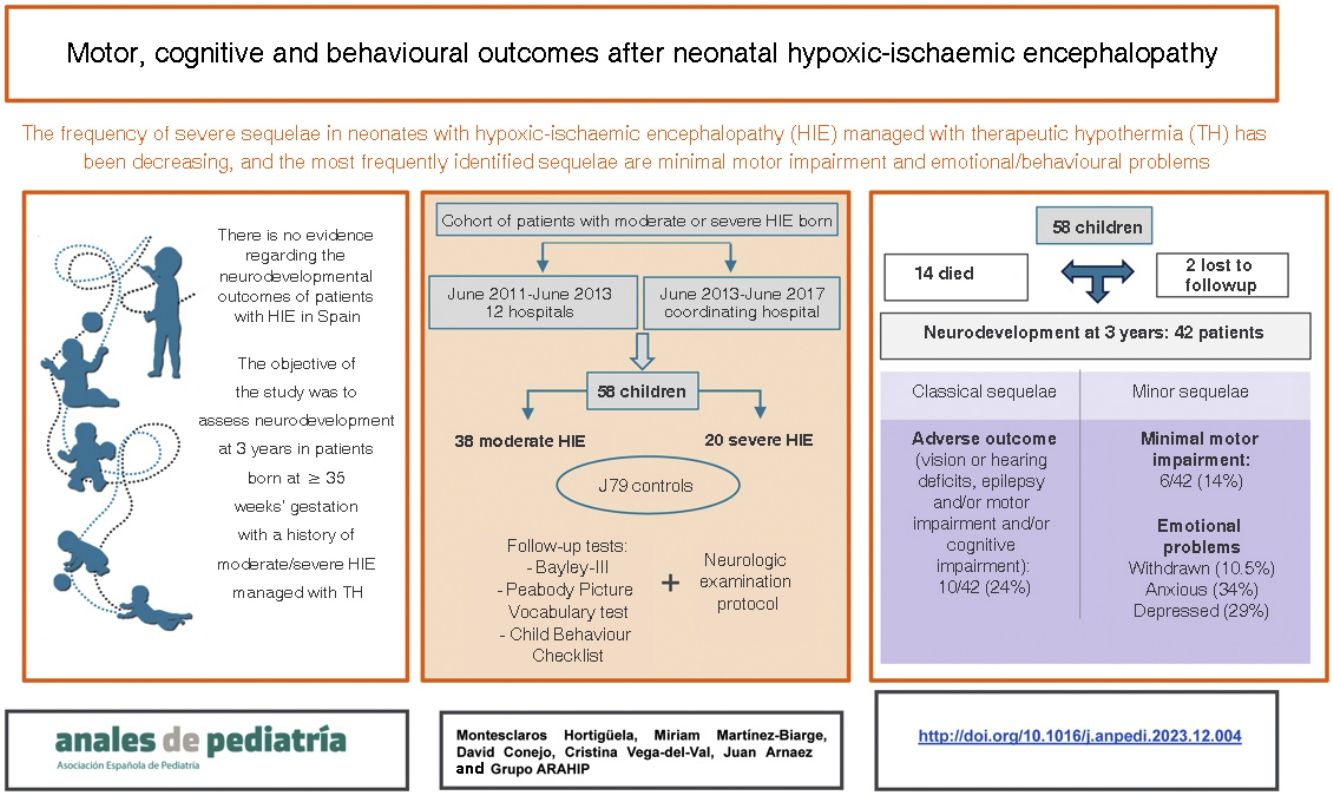
The current neurodevelopmental status of patients with neonatal hypoxic-ischaemic encephalopathy (HIE) in Spain is unknown. Recent European studies highlight a shift of severe pathology towards mild motor disorders and emotional problems. The aim of this study was to analyse neurodevelopmental outcomes in a cohort of neonates with HIE at age 3 years.
Patients and methodMulticentre observational study of neonates born at 35 or more weeks of gestation with moderate to severe HIE in 2011–2013 in 12 hospitals in a large Spanish region (91 217 m2), with the recruitment extended through 2017 in the coordinating hospital. We analysed the findings of neonatal neuroimaging and neurodevelopmental test scores at 3 years (Bayley-III, Peabody Picture Vocabulary Test and Child Behavior Checklist). The sample included 79 controls with no history of perinatal asphyxia.
ResultsSixty-three patients were recruited, of whom 5 (7.9%) were excluded due to other pathology and 14 (24%) died. Of the 44 survivors, 42 (95.5%) were evaluated. Of these 42, 10 (24%) had adverse outcomes (visual or hearing impairment, epilepsy, cerebral palsy or developmental delay). Other detected problems were minor neurological signs in 6 of the 42 (14%) and a higher incidence of emotional problems compared to controls: introversion (10.5% vs. 1.3%), anxiety (34.2% vs. 11.7%) and depression (28.9% vs. 7.8%) (P < .05). The severity of the lesions on neuroimaging was significantly higher in patients with motor impairment (P = .004) or who died or had an adverse outcome (P = .027).
ConclusionIn addition to classical sequelae, the followup of patients with neonatal HIE should include the diagnosis and treatment of minor motor disorders and social and emotional problems.
El neurodesarrollo actual de pacientes con encefalopatía hipóxico-isquémica (EHI) neonatal en España se desconoce. Recientes estudios europeos destacan el desplazamiento de la patología grave hacia trastornos motores leves y problemas emocionales. El objetivo de este estudio fue analizar el estado neuroevolutivo integral a los 3 años de una cohorte de neonatos con EHI.
Pacientes y métodosEstudio observacional multicéntrico de neonatos ≥35 semanas de edad gestacional con EHI moderada-grave nacidos entre 2011–2013 en 12 hospitales de una extensa región española (91.217 m2) y ampliado hasta 2017 en el hospital coordinador. Se evaluaron los estudios de neuroimagen neonatal y del neurodesarrollo a los 3 años mediante Bayley-III, Peabody Picture Vocabulary Test y Child Behaviour Checklist. Se incluyeron 79 controles sin asfixia perinatal.
ResultadosSe reclutaron 63 pacientes de los cuales 5/63 (7,9%) se excluyeron por presentar otra patología; 14/58 (24%) fallecieron. De los 44 supervivientes, 42/44 (95,5%) fueron evaluados. De ellos; 10/42 (24%) presentaron evolución adversa (alteraciones visuales o auditivas, epilepsia, parálisis cerebral o retraso del desarrollo). Adicionalmente se detectaron otras alteraciones: trastorno motor mínimo en 6/42 (14%) y más problemas de introversión (10,5% vs. 1,3%), ansiedad (34,2% vs. 11,7%) y depresión (28,9% vs. 7,8%) que los controles (p < 0,05). La gravedad de las lesiones en neuroimagen fue significativamente mayor en pacientes con trastorno motor (p = 0,004) y muerte o evolución adversa (p = 0,027)
ConclusionesAdemás de las secuelas clásicas, el seguimiento de los pacientes con EHI neonatal debería incluir el diagnóstico y manejo de trastornos motores mínimos y problemas emocionales.
In the management of moderate or severe hypoxic-ischaemic encephalopathy (HIE), therapeutic hypothermia (TH) has significantly changed the prognosis of affected newborns, and is currently considered the standard of care in high-income countries.1–3 Some cohort studies conducted in Europe have suggested that outcomes could be even better compared to the reports of the original clinical trials, although the reasons for this are not well understood.4,5
Since the publication of specific recommendations for the follow-up of HIE in 2014,6 we do not know of any population-based study assessing the medium- to long-term neurological outcomes of newborns with moderate or severe HIE managed with TH in Spain. On the other hand, some recent studies conducted in other countries have highlighted the presence of other neurologic sequelae, such as emotional or behavioural problems, in addition to abnormalities in more widely studied areas, such as motor or cognitive development.7,8
Obtaining up-to-date population-based data on outcomes in different areas of neurodevelopment in children with a history of HIE managed with TH in Spain is important for the purpose of establishing efficient management and follow-up programmes. In addition, comparing data from cohorts from different countries and with different health care models allows the investigation of potential risk or protective factors in relation to HIE.
The aim of our study was to analyse outcomes in every area of neurodevelopment at age 3 years in a cohort of newborns delivered at or after 35 weeks of gestation and with a diagnosis of moderate or severe HIE between 2010 and 2017 in one region in Spain.
Patients and methodsStudy populationThis study was conducted in the framework of a larger population-based study designed to improve the care of newborns with perinatal asphyxia in a region of Spain with a land area of 91 217 m2.9 It was a multicentre observational cohort study that included live neonates born at or after 35 weeks of gestation with birth weights of 1800 g or greater and moderate or severe HIE between June 2011 and June 2013 in 12 hospitals (5 level III, 5 level II and 2 level I). The coordinating hospital extended the recruitment period through December 2017, applying the same study protocol.
The definition of moderate or severe HIE was fulfilment of one A criterion as well as the B criterion. The A criteria included: (1) Umbilical cord blood pH or blood pH in the first hour post birth of 7.00 or lower; (2) 5-minute Apgar score of 5 or lower and (3) need of advanced life support (intubation, cardiac massage and/or ventilation beyond 5 min post birth). The B criterion was clinical presentation compatible with moderate or severe HIE, defined as an altered level of consciousness (lethargy, stupor or coma) in the first 6 h post birth and staged with the modified Sarnat grading scale.10 To guarantee the rigour of this classification, we recorded the neurologic exams with the consent of the parents. When the evaluation was complete, 2 neonatal neurologists (A.GA, J.A.) who were blinded to the neurodevelopmental outcomes watched the video recordings to assess the severity of HIE.11
Newborns with moderate or severe HIE received servo-controlled whole-body TH (Tecotherm TSmed 200 N or CritiCool, MTRE Ltd) with a target core temperature of 33–34 °C for 72 h, according to the national guidelines in Spain.12 Patients managed with TH were monitored with amplitude integrated electroencephalography (aEEG) during cooling.
Decisions to withdraw or withhold life-sustaining treatment were based on a combination of the following findings: persistent coma, abnormal aEEG past 48 h post birth, detection of severe abnormalities in neuroimaging (cranial ultrasound and/or brain MRI); in some cases, the levels of neuron-specific enolase were measured in cerebrospinal fluid.13
We excluded neonates with polymalformative syndrome, inborn errors of metabolism, perinatal stroke, neuromuscular disease or spine lesions from the analysis.
Magnetic resonance imagingIt was performed on a 1.5T scanner. At minimum, the MRI protocol included the acquisition of axial and sagittal T1-weighted images, coronal T2-weighted images and axial diffusion-weighted images. Two blinded researchers (M.M.-B., J.A.) independently interpreted the MRI findings based on the scoring system proposed by Rutherford et al.,14 the presence and severity of lesions in the basal ganglia/thalami (BGT), white matter (WM) and cortex, and the signal of myelination in the posterior limb of internal capsule (PLIC). The total injury score (TIS) and WM × BGT were calculated as described by Thoresen et al.15 Discrepancies between the 2 researchers were resolved by consensus.
NeurodevelopmentThe evaluations were carried out by a single researcher (M.M.H.), a paediatric neurologist specifically trained for the purpose, who was blinded to the severity of HIE and the MRI findings. Patients underwent assessment at 36 months through a structured neurologic examination and the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III), which yield composite scores (standard scores with a mean of 100 and a standard deviation [SD] of 15) for cognitive and motor skills.16 Language skills were assessed by means of the Peabody Picture Vocabulary Test, Third Edition (PPVT-III) Spanish version (standard scores with a mean of 100 and a SD of 15).17
In patients who could not be assessed before age 42 months, the cognitive evaluation was performed with the Spanish version of the Wechsler Preschool and Primary Scale of Intelligence III (WPPSI III)18 (standard scores with a mean of 100 and a SD of 20). In this group, motor skills were assessed with the Movement Assessment Battery for Children, Second Edition (MABC-2),19 calculating the percentile corresponding to each of the scores.20
The diagnosis of cerebral palsy (CP) was based on the criteria applied by the Surveillance of Cerebral Palsy in Europe network,21 and its severity assessed by means of the Gross Motor Function Classification System (GFMCS).22
We defined unfavourable outcome as death or adverse neurodevelopmental outcome. We defined adverse outcome as the presence of visual or hearing deficits, epilepsy and/or motor impairment (any patient with CP and/or a Bayley-III motor composite score <85 or a MABC-2 score ≤15th percentile) and/or cognitive impairment (Bayley-III cognitive composite score <85, intelligence score <85 in the PPVT-III or a full-scale intelligence quotient [IQ] score <80 in the WPPSI-III or WISC-IV).
Beyond the definition of adverse neurodevelopmental outcome, we assessed other neurodevelopmental problems, such as minimal motor impairment (MMI) and affective or behavioural problems. Minimal motor impairment was defined as absence of CP and at least 2 of the following: abnormal gait, dystonic posture, dystonic orofacial movements, intention tremor, difficulties in gross or fine motor skill coordination. Behavioural problems were assessed with the Child Behaviour Checklist (CBCL) for ages 1.5–5.23
We conducted telephone interviews with two families by means of a structured questionnaire asking about the general health and developmental milestones in the child. We also obtained follow-up health records from the hospital managing the child. The outcomes were classified as normal or adverse, without assigning scores.
Control groupWe recruited an unmatched control group by convenience sampling comprising 79 term neonates who did not have perinatal asphyxia (as defined above), were not admitted to hospital in the neonatal period and with a normal physical evaluation before discharge from the maternity ward. The control group was recruited in the coordinating hospital and evaluated at age 36 months with the same protocol applied to the cases.
All the evaluations (of participants with HIE and controls) took place before the COVID-19 pandemic.
Statistical analysisWe have summarised qualitative data as absolute and relative frequencies and quantitative data as median and interquartile range (IQR) or mean and standard deviation SD. We compared continuous variables by means of the Mann–Whitney U test or the Kruskal-Wallis test, as applicable. For categorical variables, we used the χ2 test or the Fisher exact test. We calculated the Spearman correlation coefficient (rs) to assess the correlation between quantitative variables. To determine the optimal MRI score cut-off point for the prediction of adverse outcomes, we analysed receiver operating characteristic (ROC) curves and calculated the area under the curve (AUC), sensitivity (Sen), specificity (Spe) positive predictive value (PPV) and negative predictive value (NPV). We also developed predictive models for the rest of neonatal variables. We considered P values of less than 0.05 statistically significant, and the analysis was performed with the statistical package SPSS version 20 (IBM, Armonk, NY, USA).
Ethical considerationsWe obtained the signed consent of parents, and the study was approved by the Clinical Research Ethics Committee (file 1243).
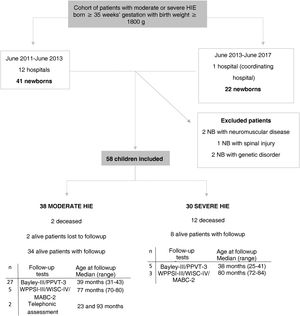
ResultsWe recruited 63 patients with moderate to severe HIE consecutively. Five were excluded, 1 due to spinal lesion, 2 due to neuromuscular disease and 2 due to a genetic disorder (Fig. 1). The main perinatal characteristics of the 58 newborns included in the sample (38 con moderate HIE and 20 with severe HIE) can be found in Supplemental Table S1 (Appendix B).
Flow diagram of the cohort of patients with moderate or severe HIE.
Bayley-III, Bayley Scales of Infant and Toddler Development, Third Edition GA, gestational age; HIE, hypoxic-ischaemic encephalopathy; IQR, interquartile range; MABC-2: Movement Assessment Battery for Children, Second Edition; max, maximum follow-up age; min, minimum follow-up age; NB, newborn; PPVT-3: Peabody Picture Vocabulary Test, third edition (version in Spanish); WISC-IV: Wechsler Intelligence Scale for Children, Fourth Edition; WPPSI-III, Wechsler Preschool & Primary Scale of Intelligence, Third Edition.
Forty-nine newborns received TH (84%), reaching a target core temperature of 33–34 °C at a median age of 1 h (IQR, 1–4.2). Nine neonates were not cooled: 2 due to instability precluding transport and 7 due to diagnosis of mild HIE, although the experts that reviewed the video recordings after the fact noted that these infants had moderate HIE. All newborns managed with TH, except 2 who were in a coma, received sedation during cooling.
Fourteen neonates died (24%), at a median of 22 h post birth (IQR, 22–87), all in the neonatal period except one who passed away at 4 months. Of the 14 deceased infants, 12 had severe HIE. In 10 of these patients, death followed the decision to withdraw or withhold life-sustaining treatment, and in 4, because of failure of intensive treatment.
Neurodevelopmental outcomesThe 42 survivors, with the exception of 2, were evaluated at a median age of 39.5 months (IQR, 38–42.3): 32 with the Bayley-III and PPVT-3, 8 with the WPPSI-III or WISC-IV and the MABC-2, and 2 through a telephone interview (Fig. 1). The 79 controls were assessed with the Bayley-III and PPVT-3 at a median age of 39 months (IQR, 37–41).
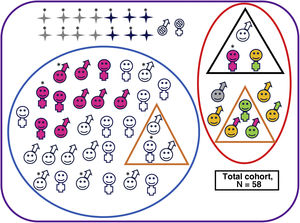
Ten of the 42 survivors of moderate or severe HIE (24%) had adverse outcomes (1/8 [12%] with severe HIE and 9/34 [26%] with moderate HIE; P = .655) (Fig. 2 and Appendix B, Supplemental Table S2), as did one control, who had language delay.
Neurodevelopmental outcomes of the overall cohort.
Deceased, male. Alive, male. Deceased, female. Alive, female. No follow-up. Adverse outcome. No adverse outcomes. Cerebral palsy. Minor motor impairment. Abnormal results in cognitive assessment (Bayley-III cognitive composite score <85 and/or PPVT-3 <85 or WPPSI-III full-scale IQ <80 or WICS-IV full-scale IQ <80). Abnormal results in motor assessment (Bayley-III motor composite score <85 or MABC-2 ≤15thpercentile).
Clinically significant scores in one or more areas of the CBCL (percentile >97th). Normal results in all developmental and behavioural tests. Patient with severe hearing loss that could not be explained by any other cause.*Patient with severe HIE.
Three of the 10 children with adverse outcomes had CP, all of whom could walk independently: 2 had GMFCS level I motor impairment and the remaining patient GMFCS level II impairment (Table 1).
Summary of clinical and radiological characteristics of patients with motor impairment (3 patients with cerebral palsy and 3 with minimal motor impairment).
| Patient | 1 | 2 | 3a | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Adverse outcome | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes |
| Type of motor impairment | CP | CP | CP | MMI | MMI | MMI | MMI | MMI | MMI |
| BAYLEY-III | |||||||||
| Cognitive composite | 100 | 120 | 100 | 95 | 95 | 125 | 90 | ||
| Motor composite | 103 | 121 | 100 | 103 | 70 | 110 | 85 | ||
| FM/GM score | 16/5 | 16/11 | 10/10 | 11/10 | 5/5 | 15/8 | 9/10 | ||
| PPVT-III. IQ | 113 | 116 | 102 | 105 | 103 | 96 | 57 | ||
| WISC-IV. Full-scale IQ | P92 | ||||||||
| MABC-2. Percentile | P9 | ||||||||
| CBCL | |||||||||
| Impulsivity | P54 | P89 | ≤ P50 | P54 | P69 | –g | ≤ P50 | P97 | |
| Anxious/depressed | ≤ P50 | P97 | P58 | P73 | P97 | P76 | ≤ P50 | P95 | |
| Somatic complaints | ≤ P50 | P79 | P79 | ≤ P50 | P62 | P87 | ≤ P50 | > P97 | |
| Withdrawn | ≤ P50 | P90 | P73 | P84 | P90 | P58 | ≤ P50 | > P97 | |
| Sleep problems | P62 | P89 | P62 | ≤ P50 | P73 | –g | ≤ P50 | P89 | |
| Attention problems | ≤ P50 | P49 | P76 | P89 | P76 | P62 | P54 | P96 | |
| Aggressive behaviour | ≤ P50 | P49 | P69 | P54 | P58 | P54 | ≤ P50 | P79 | |
| CBCL. DSM-IV | |||||||||
| Mood disorders | ≤ P50 | P84 | P58 | P54 | P58 | P58 | P54 | > P97 | |
| Anxiety | ≤ P50 | > P97 | P65 | P65 | > P97 | P90 | ≤ P50 | P96 | |
| PDD | P58 | P97 | ≤ P50 | P90 | P97 | P58 | ≤ P50 | > P97 | |
| ADHD | ≤ P50 | ≤ P50 | P76 | P84 | P76 | P76 | P54 | P58 | |
| ODD | P54 | ≤ P50 | P81 | P54 | P54 | P54 | ≤ P50 | P81 | |
| Other | –b | –b | –c | –d,e | –f | –d | – | –d | |
| MRI TIS / WM × BGT | 5/0 | 1/0 | 6/2 | 2/0 | 5/0 | 8/4 | 0/0 | 5/2 | 5/3 |
ADHD, attention-deficit hyperactivity disorder; CBCL, Child Behaviour Checklist; CP, cerebral palsy; FM, fine motor; GM, gross motor; IQ, intelligence quotient; MABC-2, Movement Assessment Battery for Children, Second Edition; MMI, minimal motor impairment; ODD, oppositional-defiant disorder; P, percentile; PDD, pervasive development disorder; PPVT-III, Peabody Picture Vocabulary Test, Third Edition; TIS, total injury score (0–11); WISC-IV, Wechsler Intelligence Scale for Children, Fourth Edition; WM × BGT, product of white matter and basal ganglia/thalamus (0–9).
Scores in the abnormal range are presented in boldface.
Another 3 children with adverse outcomes received a diagnosis of MMI. In addition, 2 of them had motor delay and 1 language delay, and 2 had abnormal scores in some area of the affective and behavioural assessment (Table 1).
Of the 32 survivors who did not meet the criteria for adverse outcome, 3 received a diagnosis of MMI (Table 1). None of the controls had MMI.
The mean scores in the neurodevelopmental tests were significantly lower in patients with HIE compared to controls (Table 2).
Comparison of the mean scores in the HIE and control groups in different areas of neurodevelopment based on the Bayley-III and PPVT-III tests (n = 32), and the domains of the CBCL (n = 38), statistically significant results.
| Area of development | HIE groupa | Congrol group (n = 79) |
|---|---|---|
| Cognitive composite, mean (SD) | 100.3 (11.5) | 105.1 (10.7) |
| Motor composite, mean (SD) | 103.4 (14.1) | 107.9 (8.8) |
| Language composite, mean (SD) | 100.9 (16.4) | 108.2 (9.7) |
| CBCL: withdrawnb, n/N (%) | 4/38 (10.5) | 1/77 (1.3) |
| CBCL: anxietyc, n/N (%) | 13/38 (34.2) | 9/77 (11.7) |
| CBCL: depressedc, n/N (%). | 11/38 (28.9) | 6/77 (7.8) |
CBCL, Child Behaviour Checklist; HIE, hypoxic-ischaemic encephalopathy; PPVT-III, Peabody Picture Vocabulary Test.
Although the difference in the overall prevalence of clinically significant behavioural and emotional problems (score >97th percentile) between children with HIE and controls was not statistically significant, the separate analysis of each domain revealed more frequent introversion (P = .04) in patients with HIE. In addition, when we considered scores at the upper limit of normal (93rd to 97th percentiles), patients with HIE had depression and anxiety features more frequently than controls (P < .05) (Table 2). In addition, patients with HIE and favourable outcomes had more depression problems compared to controls (8/30 [27%] vs. 6/77 [8%]; P = .021) and more anxiety problems (10/30 [33%] vs. 9/77 [12%]; P = .008). We did not find differences in behavioural variables within the HIE group between children with adverse neurodevelopmental outcomes and children with favourable outcomes (P = .693), nor between children with and without MMI (P = 1).
We assessed whether outcomes in the group of 9 patients with moderate HIE who did not receive TH differed from the outcomes in the group of 29 patients with HIE who were cooled, and found no differences in either the frequency of adverse neurodevelopmental outcomes (P = .403) or the combined unfavourable outcome of adverse outcome or death (P = .388). We also found no differences in the frequency of cognitive impairment (P = 1), CP or MMI (P = .160) or behavioural problems (P = .372). These findings should be interpreted with caution given the small number of patients who were not cooled.
Magnetic resonance findings and neurodevelopmentThe TIS and the WM × BGT were significantly higher in both children with MMI or MMI/CP compared to children with normal motor outcomes, and in children with unfavourable outcomes (death or adverse neurodevelopmental outcome) compared to survivors with normal development (Tables 3 and 4).
Association between MRI findings and neurodevelopmental outcomes.
| Death or AO | AO | MMI/CP | Motor AO | Cognitive AO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| n | 12 | 27 | 9 | 27 | 9 | 27 | 6 | 30 | 4 | 32 |
| TIS, median (IQR) | 5 (0.3;7.8) | 1 (0;2)a | 3 (0;5.5) | 1 (0;2) | 5 (1.5;5.5) | 1 (0;2)a | 3 (0;6.5) | 1 (0;3.3) | 2.5 (0;5.8) | 1 (0;3.8) |
| WM × BGT, median (IQR) | 1 (0; 3.8) | 0 (0;0)a | 0 (0;2.5) | 0 (0;0) | 0 (0; 2.5) | 0 (0;0)b | 0 (0;2.5) | 0 (0; 0) | 1 (0;2.8) | 0 (0; 0) |
AO, adverse outcome; CP, cerebral palsy; MMI, minimal motor impairment; TIS, total injury score (0–11); WM × BGT, product of white matter and basal ganglia/thalamus (0–9).
ROC curve cut-off points with the corresponding predictive values.
| Death or AOn/N = 12/39 | AOn/N = 9/36 | MMI/CPn/N = 9/36 | Motor AOn/N = 6/36 | Cognitive AOn/N = 4/36 | |
|---|---|---|---|---|---|
| TIS | |||||
| Cut-off point | 6 | 6 | 5 | 6 | 6 |
| AUC (95% CI) | 0.72 (0.55−0.85) | 0.63 (0.45−0.78) | 0.813 (0.65−0.92) | 0.62 (0.45−0.78) | 0.54 (0.37−0.71) |
| Sen (95% CI) | 41.7 (19.3−68) | 22.2 (6.3−54.7) | 66.7 (35.4−87.9) | 33.3 (9.68−70) | 25.0 (4.56−69.9) |
| Spe (95% CI) | 96.3 (81.7−99.3) | 96.3 (81.7−99.3) | 92.6 (76.6−97.9) | 96.7 (83.3−99.4) | 93.8 (79.9−98.3) |
| PPV | 83.3 (43.6−97.0) | 66.7 (20.8−93.9) | 75 (40.9−92.9) | 66.7 (20.8−93.9) | 33.3 (6.15−79.2) |
| NPV | 78.8 (62.2−89.3) | 78.8 (62.2−89.3) | 89.3 (72.8−96.3) | 87.9 (72.7−95.2) | 90.9 (76.4−96.9) |
| WM × BGT | |||||
| Cut-off point | 2 | 3 | 3 | 4 | 3 |
| AUC (95% CI) | 0.68 (0.52−0.82) | 0.59 (0.42−0.75) | 0.66 (0.48−0.81) | 0.58 (0.40−0.74) | 0.66 (0.48−0.81) |
| Sen (95% CI) | 50 (25.4−74.6) | 22.2 (6.3−54.7) | 22.2 (6.3−54.7) | 16.7 (3−56.4) | 25.0 (4.6−69.9) |
| Spe (95% CI) | 81.5 (63.3−91.8) | 96.3 (81.7−99.3) | 96.3 (81.7−99.3) | 100 (88.6−100) | 93.8 (79.9−98.3) |
| PPV | 54.5 (28−78.7) | 66.7 (20.8−93.9) | 66.7 (20.8−93.9) | 100 (20.7−100) | 33.3 (6.15−79.2) |
| NPV | 78.6 (60.5−89.8) | 78.8 (61.9−88.3) | 78.8 (62.2−89.3) | 85.7 (70.6−93.7) | 90.9 (76.4−96.9) |
AO, adverse outcome; AUC, area under the curve; CP, cerebral palsy; MMI, minimal motor impairment; NPV, negative predictive value; PPV, positive predictive value; Sen, sensitivity; Spe, specificity; TIS, total injury score (0–11); WM × BGT, product of white matter and basal ganglia/thalamus (0–9).
The TIS was a good predictor of MMI/CP (AUC: 0.8127) and unfavourable outcome (death or adverse neurodevelopmental outcome, AUC: 0.7191). A TIS of 7 or higher predicted death or adverse outcome with a PPV of 100%, while a TIS of less than 3 had an NPV of 84%. A TIS of 5 or greater had an NPV of 89% to predict the absence of motor impairment.
We did not find significant correlations between the neuroimaging scores and neurodevelopmental outcomes in the areas of cognition or language; however, there was a correlation between higher WM × BGT values and lower scores in the Bayley-III (rs = −0.465; P = .014).
Neonatal variables and neurodevelopmentWe analysed the usefulness of three neonatal variables in the prediction of neurodevelopmental outcomes: the presence of clinical and/or electrical neonatal seizures, an abnormal aEEG tracing (low voltage, burst suppression or flat patterns) and the variable “reaching the target core temperature after 3 h post birth”. The NPVs were adequate—greater than 80%—for most variables (Appendix B, Supplemental Table S3).
DiscussionThe percentage of survivors without neurologic sequelae in our multicentre study was greater compared to the percentage reported in patients managed with TH in randomised controlled trials (RCTs),1,3,24 but similar to the percentages reported in recent observational studies.4,15,25,26 We found motor impairment (CP or MMI) in 21.4% of the patients in our cohort, but 7.1% were classified as CP, a percentage that was lower compared to patients managed with cooling in RCTs (26–36%)1,3,27 and in recent observational studies (12–17%).5,7,25,26,28,29 Furthermore, patients with CP in our study exhibited higher levels of functioning, as none had motor impairment categorised as GMFCS level III or higher, in contrast to other cohorts in which patients with CP had more severe impairment (Table 5).
Summary of motor function outcomes in patients with cerebral palsy graded according to the Gross Motor Function Classification System (GFMCS) reported in the literature, including randomised controlled trials and observational studies in patients managed with therapeutic hypothermia.
| First authorRecruitment periodGeographical region | Study design | Age interval of follow-up | CP n/total (%) | CP GMFCS III-V | CP GMFCSn/total (%) |
|---|---|---|---|---|---|
| Azzopardi D12002-2006United Kingdom | RCA | 18 months | 33/120 (28%) | Yes | GMFCS I-II: 11/120 (9%)GMFCS III-V: 24/120 (20%) |
| Jacobs S32001-2007Australia/New Zealand/Canada/USA | RCA | 24 months | 21/79 (26.6%) | Yes | GMFCS I:5/79 (6.3%)GMFCS II-V: 16/79 (20.3%) |
| Simbruner G402001−2006Germany/Austria/South Africa/Belgium/France/Singapore/Italy/Denmark | RCA | 18−21 months | No data | Yes | GMFCS III-V: 4/32 (12.5%) |
| Guillet R271999-2002USA/UK/Canada/New Zealand | RCA | 18 months | 49/135 (36.3%) | Yes | GMFCS I-II: 17/135 (12.6%)GMFCS III-V: 32/135 (23.7%) |
| Jary S52006-2012Bristol | Observational | 18−24 months | 18/93 (19.4%) | Yes | GMFCS I:12/93 (13%)GMFCS V:6/93 (6.4%) |
| Garfinkle J252008-2010Canada | Observational | 24 months | 4/26 (15%) | Yes | GMFCS II-V: 4/26 (15%) |
| Grossmann K292007-2009Sweden | Observational | 6−43 months | 10/59 (17%) | Yes | GMFCS I-II: 5/59 (8.4%)GMFCS III-V: 5/59 (8.4%) |
| Hortigüela M, et al cohort2011−2017Spain | Observational | 36 months | 3/42 (7.1%) | No | GMFCS I-II: 3/42 (7.1%)GMFCS III-V: 0/42 (0%) |
CP, cerebral palsy; GMFCS, Gross Motor Function Classification System; RCT, randomised controlled trial.
No data: this study40 only presented data for patients with cerebral palsy classified as GMFCS level III-V.
Although the reasons for this improvement are not clear, one possible explanation is the earlier initiation of TH combined with the routine use of sedation and rigorous monitoring and correction of different factors that could worsen hypoxic-ischaemic damage, such as hypoglycaemia and hypocarbia.30–32
In our cohort, 14.2% of the patients had MMI. This clinical condition could constitute a milder presentation of motor impairment thanks to the use of TH. Half of the patients with MMI also had neurodevelopmental delays, a finding reported in other studies that supports our hypothesis.7,33
The patients with moderate or severe HIE in this cohort also exhibited a lower rate of cognitive delay compared to patients managed with TH in RCTs1–3 and recent observational studies.5,25,26,34,35 This could be due to the decrease in motor impairment, as both types of sequelae tend to be associated in patients with a history of HIE.5,25,26 However, in our cohort, the mean scores in each area of development were significantly lower in patients with HIE compared to controls, as has been described by other research groups.8
Behavioural problems had already been described in patients with HIE before the introduction of TH.36,37 After the introduction of this treatment, they are still reported, especially in studies of patients without CP,7,8,28 in whom anxiety and depression have also been detected,7,28 results that are similar to those found in our cohort. These aspects should not be neglected, as anxiety and depression increase overall morbidity and mortality and have a negative impact on the quality of life of the patients.38
Despite the low frequency of patients with sequelae, which limited the analysis, our study corroborated the usefulness of MRI for prediction of neurodevelopmental problems following neonatal HIE.15,39
Another limitation of our study is the small number of patients with severe HIE, in wom neonatal mortality was as high as 60%, which precluded the collection of data about their neurodevelopmental outcomes.
The main strengths of the study were, on one hand, the recruitment of patients within a systematic surveillance programme with application of homogeneous case definitions in participating hospitals.9 Another strength was the high proportion of patients that remained in follow-up and the structured evaluation of multiples areas of neurodevelopment by a single examiner. Still, it would have been preferable if all children had been evaluated at the same age, as 20% were assessed when they were older. This study is the first to offer data on long-term neurodevelopmental outcomes in different areas of development in a Spanish cohort of newborns with HIE treated with TH.
In conclusion, our findings suggest that the reduction in the frequency of severe sequelae and the lower proportion of disability or impairment in newborns with moderate or severe HIE reported in cohorts in neighbouring countries is also occurring in Spain.
However, despite the reduction in severe sequelae, the neurodevelopment of children with a history of HIE cannot be compared to that of the controls, as their mean scores were lower for every area of development and a greater proportion presented motor impairment or affective or behavioural problems. For this reason, newborns with HIE require rigorous and standardised follow-up, preferably until school age, including evaluations for detection of these emerging neurodevelopmental profiles.33
Further studies on the subject need to be conducted in Spain to obtain an adequate overview of the current problems and needs of neonates with HIE. This would allow health care providers to offer more accurate prognoses to families, and make more efficient follow-up plans and use of the social, health care and education resources required by this population.
FundingFundación Ernesto Sánchez-Villares (No. FESV9/2014).
Fundación Burgos para la Investigación de la Salud (No. 1632).
Grant for Year 5 training in Paediatric Neurology given by the Sociedad Española de Neurología Pediátrica (SENEP).
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Sara Calvo (Fundación Burgos por la Investigación de la Salud) for her help in the statistical analysis.
Elena Pilar Gutiérrez. Neonatal Unit, Hospital Universitario de Salamanca, Salamanca, Spain.
Sonia Caserío. Neonatal Unit, Hospital Universitario Río Hortega, Valladolid, Spain.
María Pilar Jiménez. Neonatal Unit, Hospital Nuestra Señora de Sonsoles, Ávila, Spain.
Leticia Castañón. Neonatal Unit, Hospital Universitario de León, León, Spain.
Inés Esteban. Neonatal Unit, Hospital San Pedro, Logroño, Spain.
Miryam Hortelano. Neonatal Unit, Hospital General de Segovia, Segovia, Spain.
Natalio Hernández. Department of Paediatrics, Hospital General de Zamora, Zamora, Spain.
Marisa Serrano. Neonatal Unit, Hospital Santa Bárbara, Soria, Spain.
Tere Prada. Neonatal Unit. Hospital El Bierzo, Ponferrada, León, Spain.
Pablo Diego. Department of Paediatrics, Hospital Santiago Apóstol, Miranda de Ebro, Burgos, Spain.
Florentino Barbadillo. Department of Paediatrics, Hospital Santos Reyes, Aranda de Duero, Burgos, Spain.
Appendix A lists the members of the ARAHIP Group.
Previous meeting: the design and objectives of this study (but not its results) were presented as an oral communication at the XL Annual Meeting of the Sociedad Española de Neurología Pediátrica (SENEP), held May 25–27, 2017 in Madrid, Spain, where it received an award.