Hydrops fetalis (HF) is a rare condition with a high mortality. This study analysed the obstetric and perinatal outcomes of antenatally diagnosed HF according to its aetiology and the possibility of intrauterine treatment (IUT).
Patients and methodsWe carried out a retrospective review of the health records of 164 pregnant women with a prenatal diagnosis of HF in a tertiary care centre between 2011−2021. We analysed prenatal interventions, clinical findings, aetiologies and obstetric and live-born infant outcomes.
ResultsAn invasive prenatal study had been performed in 79.3% cases. The most common aetiologies were genetic disorders (31%), TORCH and parvovirus B19 infections (9.7%) and structural heart diseases (9.1%). Intrauterine treatment was performed in 25.6%, and 74.4% of pregnancies were terminated. Pregnancies with a prenatal diagnosis of genetic or chromosomal disorders had higher rates of elective termination compared to other aetiologies (P < .01). Among all pregnancies, only 25.6% resulted in live births (LBs), most of them preterm. Perinatal and 1-year survival rates were higher in the group that received IUT (P < .001). Among the LBs, structural heart diseases had the worst survival rates, while the aetiology with the best outcomes was tachyarrhythmia. Survival at 1 year of life among those born alive was 70%, but 58.6% of these infants had significant morbidity at discharge.
ConclusionsDespite advances in the management of FH, the poor obstetric prognosis, perinatal mortality and morbidity of survivors is still significant. These data are important for the purpose of counselling families when HF is diagnosed antenatally.
El hidrops fetal (HF) es una condición rara con una alta mortalidad. Este estudio analiza la evolución obstétrica y perinatal de los diagnósticos prenatales de HF, relacionándola con la etiología y el tratamiento intrauterino (TIU) recibido.
Pacientes y métodosSe revisaron 164 gestantes con diagnóstico prenatal de HF entre 2011 y 2021. Se registraron intervenciones prenatales, hallazgos clínicos, etiologías y resultados de los recién nacidos vivos.
ResultadosSe realizó estudio invasivo prenatal en 79,3% de pacientes. Las etiologías mayoritarias fueron alteraciones genéticas (31%), infecciones TORCH y por parvovirus B19 (9,7%) y cardiopatías estructurales (9,1%). En 25,6% se realizó TIU y entre todas las gestaciones un 74,4% fueron interrumpidas. Las alteraciones genéticas tuvieron tasas más altas de interrupción legal del embarazo respecto a otras etiologías (p < 0,01). Del total, solo nacieron un 25,6% de los fetos, la mayoría pretérmino. Aquellos que recibieron TIU gozaron de mayores tasas de supervivencia perinatal y al año de vida (p < 0,001). De entre aquellos nacimientos, las cardiopatías estructurales presentaron las peores tasas de supervivencia mientras que las causas con mejor pronóstico fueron las taquiarritmias. La supervivencia al año de vida entre aquellos recién nacidos vivos fue del 70% pero el 58,6% asoció morbilidad significativa al alta.
ConclusionesA pesar de los avances en el manejo del HF, el mal pronóstico obstétrico, la mortalidad perinatal y la morbilidad de los supervivientes siguen siendo significativos. Estos datos son importantes para asesorar a las familias que reciben un diagnóstico prenatal de HF.
Hydrops fetalis (HF) is an infrequent foetal disease. Depending on its pathophysiology, there are two types of HF: immune HF and non-immune HF (NIHF), the latter of which may be secondary to multiple structural or physiological abnormalities. Due to the generalised use of anti-D gamma globulin prophylaxis, nearly 90% of cases diagnosed at present have a non-immune aetiology, with an approximate prevalence of 1/1700 to 1/3000 pregnancies.1,2
It is a severe disease with a poor prognosis, with a reported perinatal mortality that ranges between 50% and 98%.3–5 The prenatal and neonatal prognosis, however, vary depending on the underlying aetiology, the possibility of initiating intrauterine treatment, the gestational age at the time of diagnosis and the severity of HF.
Some authors have attempted to describe the prenatal and postnatal prognosis of antenatally diagnosed HF based on the underlying aetiology,6–8 but there is scarce evidence analysing the prognosis of HF overall and based on the possibility of providing IUT. Similarly, few studies have assessed long-term survival in infants product of such pregnancies. Knowing the foetal prognosis and neonatal outcomes depending on the underlying aetiology is essential in order to counsel families in the event of a diagnosis of HF.
In consequence, the objective of our study was to analyse medium- and long-term obstetric and perinatal outcomes for pregnancies with an antenatal diagnosis of HF, with an immune or non-immune aetiology, in our hospital, and their association with the aetiology of HF and the delivered IUT. We also sought to identify prenatal and perinatal outcome predictors that could be useful for antenatal counselling.
Material and methodsWe conducted a retrospective observational study in pregnant women that received a prenatal diagnosis of HF between January 1, 2011 and December 31, 2021 who received prenatal and postnatal care at our hospital.
The sample included the pregnancies with a prenatal diagnosis of HF and the live neonates product of these pregnancies if the prenatal and postnatal care was delivered at our hospital during the study period, whether the infants had hydrops at the time of birth or not.
We excluded newborns for whom the data were incomplete due to transfer to a different facility in the prenatal or postnatal period. We also excluded pregnant women with an isolated diagnosis of ascites, pleural effusion or hydrothorax that did not meet the criteria for HF.
Hydrops fetalis was defined as the detection on the foetal ultrasound examination of subcutaneous oedema associated with effusion in a serous cavity or an abnormal fluid collection in two or more serous cavities (pericardium, pleura or peritoneum).
We defined intrauterine treatment (IUT) as any medical or surgical intervention performed during pregnancy with the aim of treating the underlying cause of hydrops or ameliorate its impact on the foetus. The definition comprehended pharmacological treatment (eg antiarrhythmic medication), transfusion of blood products, foetoscopic procedures (such as laser coagulation of placental anastomoses or serous cavity aspiration and drainage) as well as foetal surgery. The aetiologies that can be treated medically are foetal tachyarrhythmias and anaemias. Laser photocoagulation or umbilical cord occlusion was performed in monochorionic pregnancies that meet the criteria for severe twin-twin transfusion syndrome with significant impact on one of the foetuses. Foetal surgery was contemplated in the case of malformations or tumours that could benefit from IUT, and palliative treatment of HF consisted of placement of drainage lines or aspiration of cavities in foetuses in cases in which the fluid accumulation had a significant impact on the foetus.
Neonatal death was defined as death between 0 and 28 days post birth. We defined morbidity as any significant disease, sequela or impairment beyond 1 year post birth. The definition of cardiac morbidity included chronic heart failure, haemodynamically significant structural heart disease, arrhythmia requiring prescription of pharmacological treatment or electrophysiology procedures at discharge or pulmonary hypertension at the time of discharge. The definition of neurologic morbidity included psychomotor delay, moderate to severe motor impairment, hearing loss, blindness, attention-deficit hyperactivity disorder, language disorder, dysphagia or epilepsy. Lastly, the definition of nephrological morbidity included hypertension requiring pharmacological treatment and chronic kidney disease. The followup after discharge took place within the period under study, lasting between 1 and 10 years.
We reviewed the health records of each patient through the electronic health record system of the hospital, collecting obstetric data (maternal characteristics, trimester at diagnosis, ultrasound findings, indication for and type of invasive prenatal test, cause of HF, detected genetic changes, need of IUT), data about the birth (gestational age at birth, birth weight, presence of hydrops at birth, characteristics of the delivery and type of resuscitation required, need of cavity drainage and need of admission in the neonatal intensive care unit [NICU]) and data on neonatal outcomes (type and duration of respiratory support, need of cavity drainage, parenteral nutrition or dialysis, length of stay in the NICU, comorbidities in the medium and long term, death and timing of death in days post birth).
All the information was collected in a database built with the software SPSS, version 25.0. We made an initial descriptive analysis to determine the frequencies, measures of central tendency and measures of dispersion. In the inferential analysis, we used different tests depending on the nature of the variables (χ2 test, Fisher exact test, Student t-test, Mann-Whitney U de Mann-Whitney). We considered P values of less than .05 statistically significant.
The study was approved by the research ethics committee of the hospital (PR[AMI] 46/2023).
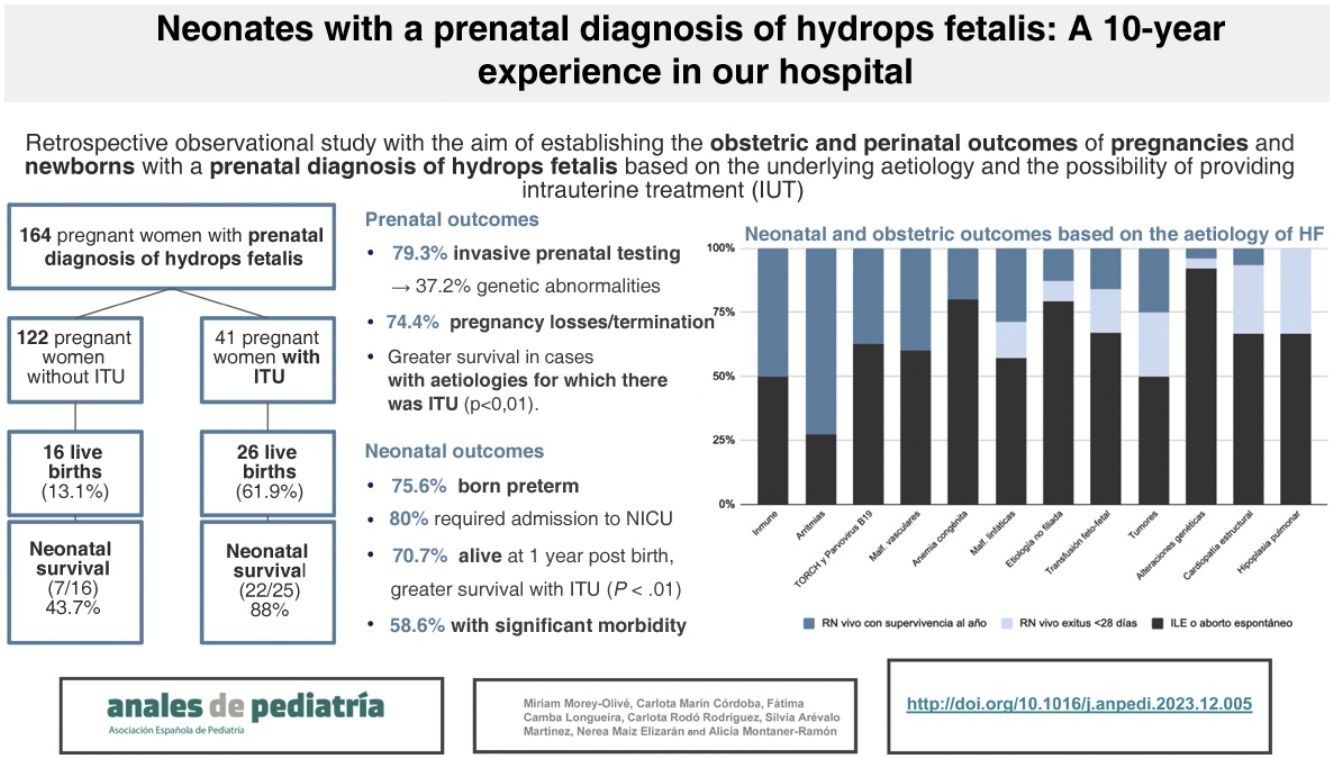
ResultsPrenatal courseDuring the 10-year period, the hospital managed 164 pregnant women with an antenatal diagnosis of HF. Nine (5.5%) were lost to followup at the end of the pregnancy due to transfer to other centres.
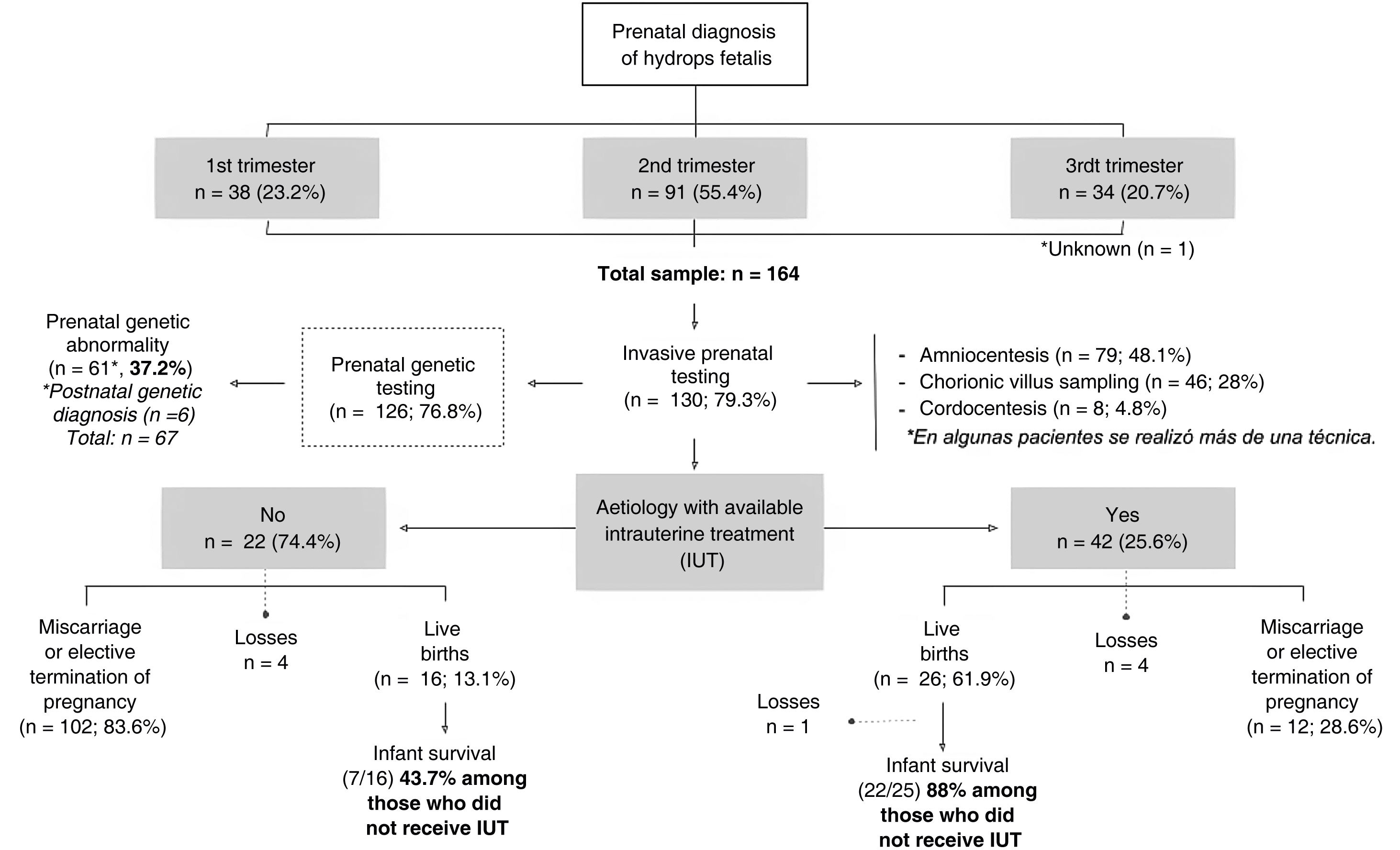
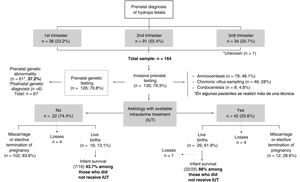
Fig. 1 presents a flow diagram of the diagnosis, treatment and outcomes of the patients included in the sample.
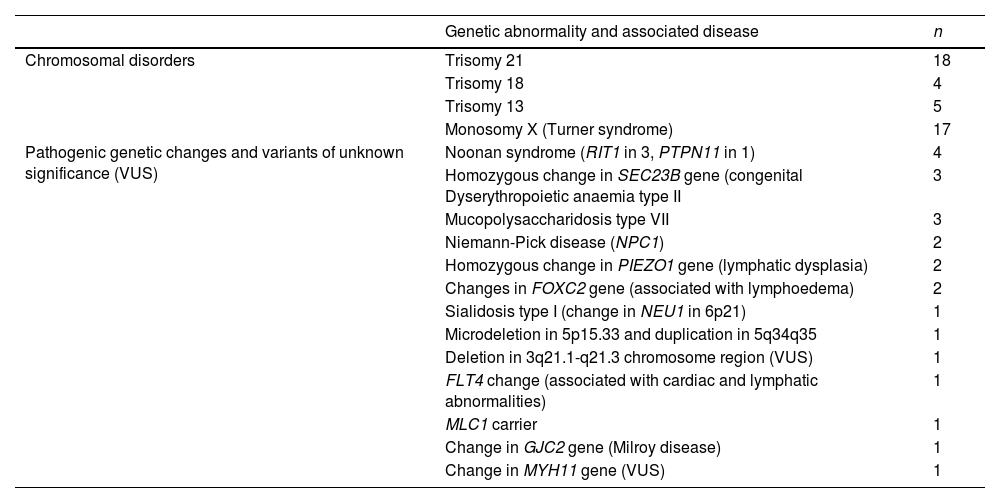
In most pregnancies, genetic testing was performed antenatally (76.8%), and it the rest it was not conducted on account of miscarriage or the decision to terminate the pregnancy. Overall, genetic changes were detected in 67 patients (40.8%) (Table 1), in some cases after birth, or even after the death of the patient.
Genetic changes identified in the total cohort.
| Genetic abnormality and associated disease | n | |
|---|---|---|
| Chromosomal disorders | Trisomy 21 | 18 |
| Trisomy 18 | 4 | |
| Trisomy 13 | 5 | |
| Monosomy X (Turner syndrome) | 17 | |
| Pathogenic genetic changes and variants of unknown significance (VUS) | Noonan syndrome (RIT1 in 3, PTPN11 in 1) | 4 |
| Homozygous change in SEC23B gene (congenital Dyserythropoietic anaemia type II | 3 | |
| Mucopolysaccharidosis type VII | 3 | |
| Niemann-Pick disease (NPC1) | 2 | |
| Homozygous change in PIEZO1 gene (lymphatic dysplasia) | 2 | |
| Changes in FOXC2 gene (associated with lymphoedema) | 2 | |
| Sialidosis type I (change in NEU1 in 6p21) | 1 | |
| Microdeletion in 5p15.33 and duplication in 5q34q35 | 1 | |
| Deletion in 3q21.1-q21.3 chromosome region (VUS) | 1 | |
| FLT4 change (associated with cardiac and lymphatic abnormalities) | 1 | |
| MLC1 carrier | 1 | |
| Change in GJC2 gene (Milroy disease) | 1 | |
| Change in MYH11 gene (VUS) | 1 |
The percentage of elective termination of pregnancy (ETP) was significantly greater in women who had received an antenatal diagnosis of genetic abnormalities compared to those who had not received it (62.3% vs 39.7%; P = .015). In total, 79 pregnancies ended due to ETP (48.2%) and 34 due to miscarriage (20.7%). Of the patients with a prenatal diagnosis of HF, 25.6% were born alive (n = 42), of whom one died in the delivery room. In the subset with a prenatal diagnosis of HF and antenatal detection of a genetic abnormality, 14.7% were born alive (n = 9).
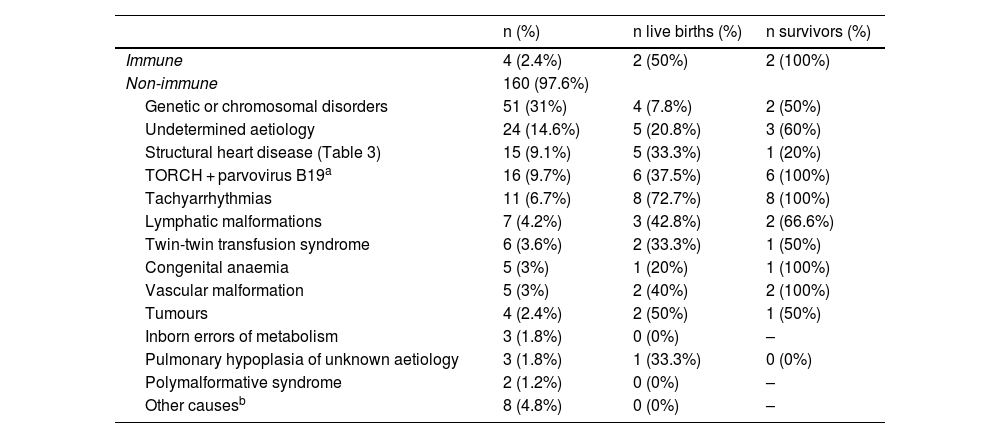
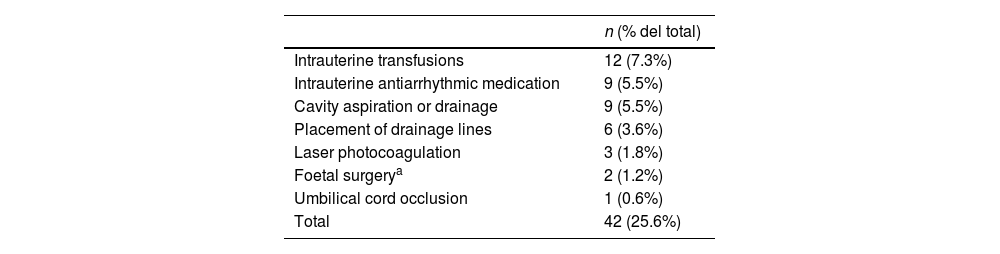
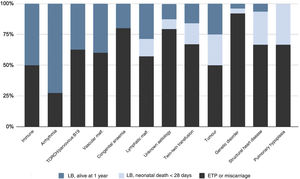
Outcomes based on the aetiology and intrauterine treatmentTables 2 and 3 present the causes to which HF was attributed in our study. All pregnant women with aetiologies that could benefit from IUT (n = 42; 25.6% of the sample) received the indicated treatment (Table 4).
Underlying aetiology of hydrops fetalis: live births and long-term survivors by aetiology.
| n (%) | n live births (%) | n survivors (%) | |
|---|---|---|---|
| Immune | 4 (2.4%) | 2 (50%) | 2 (100%) |
| Non-immune | 160 (97.6%) | ||
| Genetic or chromosomal disorders | 51 (31%) | 4 (7.8%) | 2 (50%) |
| Undetermined aetiology | 24 (14.6%) | 5 (20.8%) | 3 (60%) |
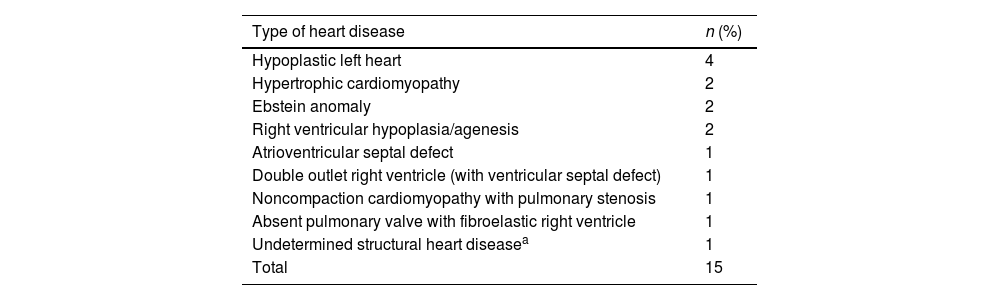
| Structural heart disease (Table 3) | 15 (9.1%) | 5 (33.3%) | 1 (20%) |
| TORCH + parvovirus B19a | 16 (9.7%) | 6 (37.5%) | 6 (100%) |
| Tachyarrhythmias | 11 (6.7%) | 8 (72.7%) | 8 (100%) |
| Lymphatic malformations | 7 (4.2%) | 3 (42.8%) | 2 (66.6%) |
| Twin-twin transfusion syndrome | 6 (3.6%) | 2 (33.3%) | 1 (50%) |
| Congenital anaemia | 5 (3%) | 1 (20%) | 1 (100%) |
| Vascular malformation | 5 (3%) | 2 (40%) | 2 (100%) |
| Tumours | 4 (2.4%) | 2 (50%) | 1 (50%) |
| Inborn errors of metabolism | 3 (1.8%) | 0 (0%) | – |
| Pulmonary hypoplasia of unknown aetiology | 3 (1.8%) | 1 (33.3%) | 0 (0%) |
| Polymalformative syndrome | 2 (1.2%) | 0 (0%) | – |
| Other causesb | 8 (4.8%) | 0 (0%) | – |
Structural heart diseases.
| Type of heart disease | n (%) |
|---|---|
| Hypoplastic left heart | 4 |
| Hypertrophic cardiomyopathy | 2 |
| Ebstein anomaly | 2 |
| Right ventricular hypoplasia/agenesis | 2 |
| Atrioventricular septal defect | 1 |
| Double outlet right ventricle (with ventricular septal defect) | 1 |
| Noncompaction cardiomyopathy with pulmonary stenosis | 1 |
| Absent pulmonary valve with fibroelastic right ventricle | 1 |
| Undetermined structural heart diseasea | 1 |
| Total | 15 |
Provided intrauterine treatment.
| n (% del total) | |
|---|---|
| Intrauterine transfusions | 12 (7.3%) |
| Intrauterine antiarrhythmic medication | 9 (5.5%) |
| Cavity aspiration or drainage | 9 (5.5%) |
| Placement of drainage lines | 6 (3.6%) |
| Laser photocoagulation | 3 (1.8%) |
| Foetal surgerya | 2 (1.2%) |
| Umbilical cord occlusion | 1 (0.6%) |
| Total | 42 (25.6%) |
Of the pregnant women with HF with aetiologies for which IUT was indicted, 61.9% had live newborns, compared to 13.1% of cases with aetiologies that could not benefit from IUT (P < .001). The percentage of neonatal survival was greater in live newborns product of pregnancies in which IUT was provided compared to pregnancies without this treatment (88% vs 43.7%; P < .01).
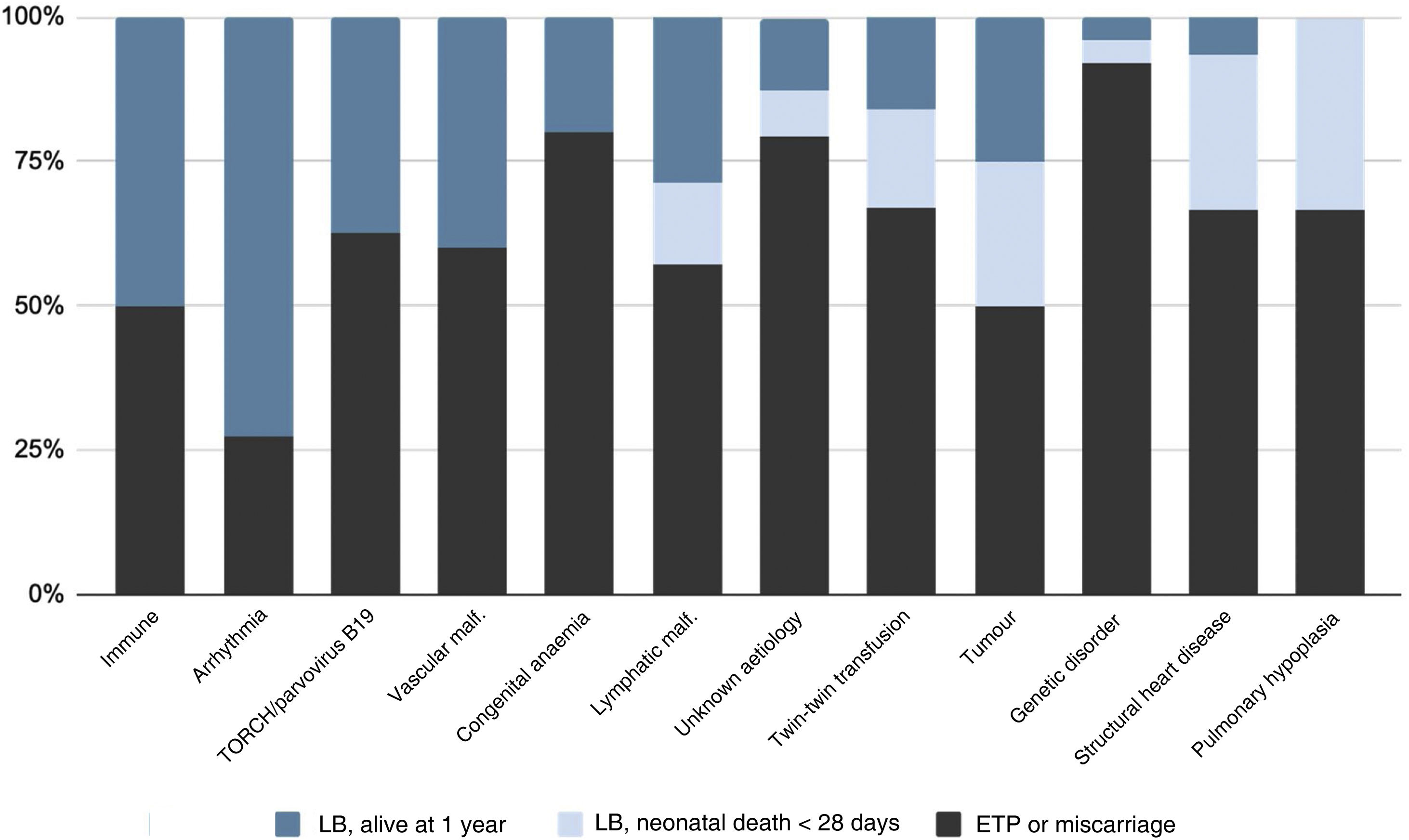
Fig. 2 summarises the pregnancy and neonatal outcomes based on the aetiology of HF. The pregnancies with the highest proportion of live births were those corresponding to diagnoses of tachyarrhythmias. The survival in newborns with a history of isoimmunization, tachyarrhythmia, TORCH infection or parvovirus B19 infection, vascular malformations or congenital anaemia was 100%. Among the live newborns, excluding those with infrequent aetiologies (Table 2), the aetiologies with the poorest neonatal prognosis were structural heart diseases, with a survival of 20%.
Perinatal outcomes among live newbornsThe mean gestational age was 33+6 weeks (range, 27+1–40+6 weeks), and 75.6% of infants were born preterm (24.4% before 32 weeks). The mean birth weight of live newborns was 2495 g (range, 810−3840 g). Of all live newborns, 58.5% had hydrops at birth.
Of the 41 live newborns (excluding the one who died in the delivery room), 80% were admitted to the NICU. The median length of stay in the NICU was 39 days (range, 1–121). Of the newborns who had hydrops at birth, 31.7% required thoracocentesis (4 in the delivery room, 9 in the NICU), 29.2% paracentesis (7 in the delivery room, 5 in the NICU) and 7.3% peritoneal dialysis (3).
Invasive mechanical ventilation was required by 60.9% of the patients, with a median duration of 9 days (range, 1–63 days), while 70.7% required some form of respiratory support (including non-invasive mechanical ventilation or oxygen therapy), with a median duration of 13 days (range, 1–108 days).
Medium- and long-term morbidity and mortality in live infantsAmong the live newborns with a prenatal diagnosis of HF, 70.7% survived in the long term (n = 29), although 58.6% had significant morbidity at discharge from followup. In the medium or long term, 14 patients had cardiovascular morbidity (48.2%), 11 had neurological morbidity (37.9%) and 1 had nephrological morbidity (3.4%). Two patients were discharged home with at-home respiratory support (6.8%), and none of the patients in the cohort required performance of tracheostomy. We ought to highlight that 48.7% of the patients with a prenatal diagnosis of HF had abnormal neuroimaging findings during the stay in the NICU, which were not clinically significant in one third.
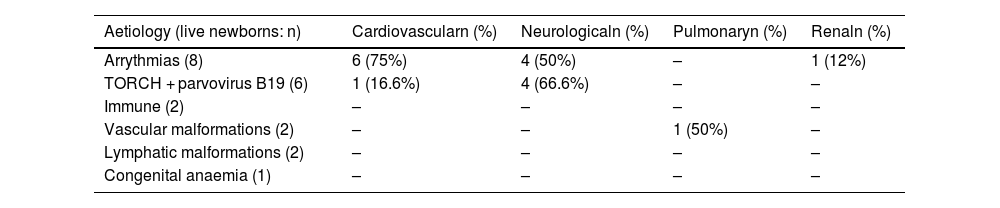
Table 5 summarises the findings regarding long-term morbidity in relation to the aetiologies associated with the greatest neonatal survival, such as arrythmias and intrauterine infections (TORCH, parvovirus B19).
Long-term morbidity in the aetiologies with the greatest neonatal survival.
| Aetiology (live newborns: n) | Cardiovascularn (%) | Neurologicaln (%) | Pulmonaryn (%) | Renaln (%) |
|---|---|---|---|---|
| Arrythmias (8) | 6 (75%) | 4 (50%) | – | 1 (12%) |
| TORCH + parvovirus B19 (6) | 1 (16.6%) | 4 (66.6%) | – | – |
| Immune (2) | – | – | – | – |
| Vascular malformations (2) | – | – | 1 (50%) | – |
| Lymphatic malformations (2) | – | – | – | – |
| Congenital anaemia (1) | – | – | – | – |
Our study showed that HF is associated with a high mortality overall, and particularly with a high prenatal mortality. In addition, the prevalence of postnatal morbidity among live newborns was also substantial. We also found that survival was, overall, higher in pregnancies with HF due to causes for which IUT was available, on account not only of a larger proportion of live births but also of a greater survival at 1 year post birth compared to cases of HF that could not be treated in utero.
The current literature on HF is scarce and the results of different studies are often contradictory. When it came to prenatal mortality, our findings were consistent with most other case series, in which the proportion of live births is of approximately 20%–25%, with the exception of a few series, like the one published by Meng et al.,6 in which the proportion of live births was higher. There is substantial variation between studies in the reported survival of live newborns, which ranges from 25% to 90.4%, and was 70.7% in our cohort.6–8. These differences probably stem from the heterogeneity of the studies, as most only include cases of NIHF, and significant differences in the laws regarding termination of pregnancy in the countries where the studies have been conducted, which could affect study outcomes.
The most frequent underlying causes in our series were chromosomal disorders and gene changes, followed by infection and cardiopathies (including tachyarrhythmias and structural heart diseases). These findings are consistent with the existing literature, although some studies have reported higher proportions of cardiac involvement and lower proportions of genetic disorders.8–10 The latter could be related to advances in genetic and molecular diagnostic techniques in recent years in Spain, which have improved the detection of monogenic disorders associated with NIHF. In 2020, Quinn et al.11 published a systematic review in which they identified 131 genes for which there was strong evidence supporting an association with NIHF. In our sample, single gene changes were identified in 21 patients, most of them variants previously associated with NIHF in the literature.
We also ought to highlight that, despite the advances in diagnostic and genetic techniques,12 there is still a significant percentage of idiopathic HF cases. In our cohort, the underlying aetiology was not identified in 1 out of 7 patients, a percentage that was lower compared to the previous literature (17.8%–34.7%).1,6,13 Differences between centres in the available diagnostic methods could explain the heterogeneity of the results.
In our study, we found a significantly greater percentage of elective termination of pregnancy in pregnant women given a prenatal genetic disorder diagnosis compared to those who did not receive such a diagnosis. Our study is the first to analyse this association and underscores the importance of improving prenatal aetiological diagnosis and the early diagnosis of genetic causes to improve prenatal counselling.
As regards prenatal treatment, one fourth of the patients in our study could receive IUT during the pregnancy, most frequently cavity drainage, intrauterine transfusions and antiarrhythmic medication, findings that were also consistent with the previous literature.1,8 An important finding in our study is that the overall outcomes were better in patients who received IUT compared to those who did not, which is particularly relevant in relation to the prenatal counselling offered to the families.
The causes of HF associated with the greatest survival, foetal as well as neonatal, were infection and arrhythmia. Every published series, including our study, has identified chromosomal and genetic disorders as the aetiologies with the poorest obstetric outcomes, due in part, as noted above, to the greater percentages of ETP and miscarriage associated to these causes. On the other hand, the causes associated with the poorest neonatal outcomes were structural heart diseases and pulmonary hypoplasia, with a survival of less than 20% at 1 year post birth, which was consistent with previous studies.14,15
The published evidence on the postnatal management and morbidity after discharge is scarce and does not allow comparisons between studies. Meng et al.6 reported a higher mortality in their case series compared to ours (77.2% versus 58.6%). Another finding worth highlighting was the considerable long-term morbidity observed in diseases associated with a lower neonatal mortality, such as intrauterine infections, arrythmias and congenital anaemias. This was particularly relevant in the case of intrauterine infections, as two thirds of the patients with this history in our study had significant neurologic morbidity in the long term. However, these findings were not statistically significant due to the limited sample size.
Among the main strengths of our study, its large sample, one of the largest case series published to date analysing obstetric and neonatal outcomes of immune or non-immune HF and the prognosis based on the possibility of delivering IUT. It is also one of the first series analysing both survival and morbidity in these patients in the medium to long term. In addition, our hospital is a reference centre for the prenatal treatment of HF, thereby minimising the risk of selection bias intrinsic to retrospective studies.
However, there are also limitations to our study, chief of which is its retrospective design. In addition, while the sample was large, the size of the groups corresponding to each of the underlying causes of HF was small, limiting the statistical power of the results and the generalization to other populations. On the other hand, the low incidence of HF made it necessary to include patients managed over a long time, and therefore there may have been significant changes both in obstetric and neonatal care during the study period.
ConclusionHydrops fetalis is a rare foetal condition associated with a high morbidity and mortality and with a heterogeneous aetiology. Since it is an infrequent and complex condition, it is essential that it is managed with a multidisciplinary approach in tertiary care hospitals to continue advancing the development of techniques allowing an early aetiological diagnosis, offer highly specialised obstetric and neonatal care and conduct prospective studies in larger allowing a more detailed analysis of obstetric outcomes, neonatal survival and long-term morbidity and mortality based on the aetiology of HF, all for the ultimate purpose of improving prenatal counselling for families in the event of diagnosis of HF.
FundingThis research project did not receive specific financial support from funding agencies in the public, private or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.