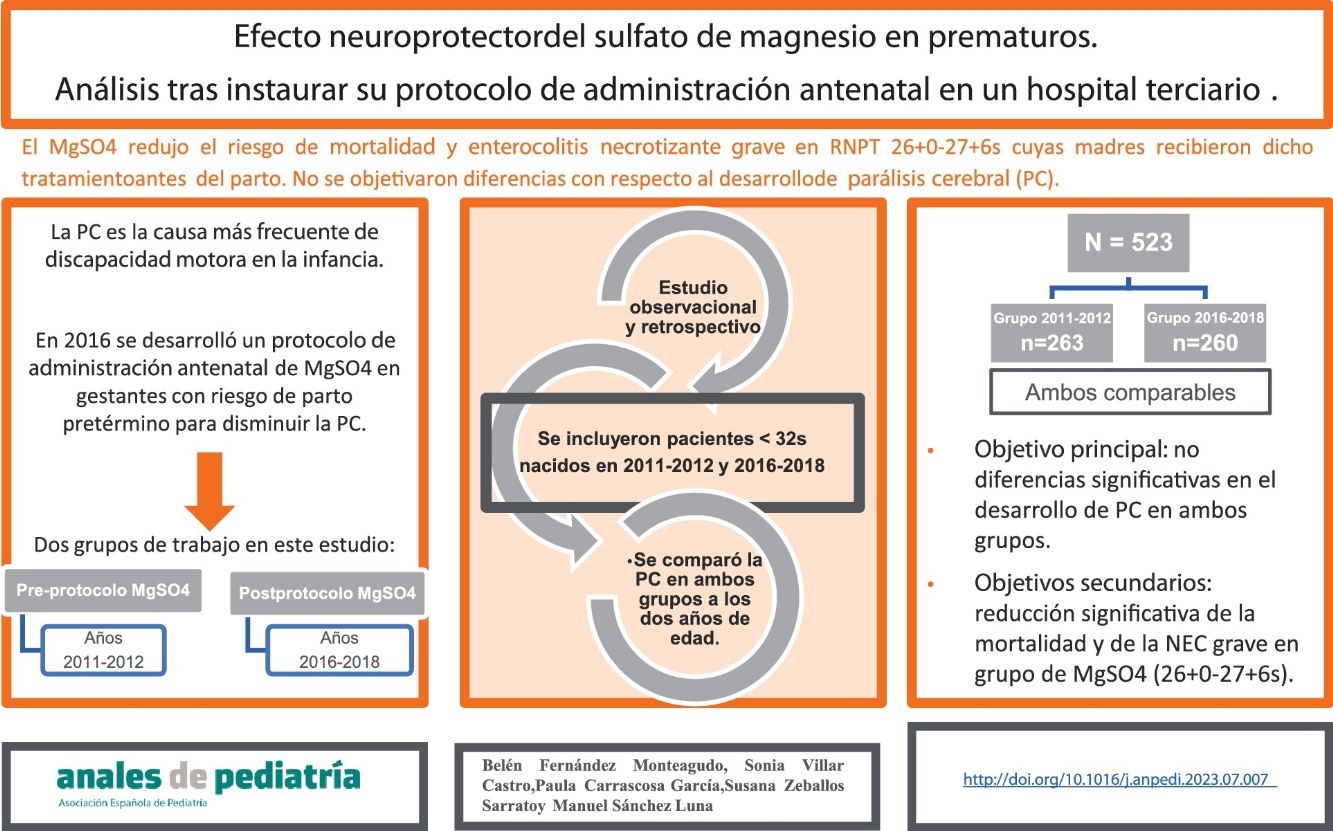
In 2016, a protocol was developed in our hospital for the antenatal administration of magnesium sulfate in pregnant women at risk of imminent preterm birth as a method to reduce the risk of cerebral palsy (CP).
Material and methodsWe conducted a retrospective observational study in a level IIIC hospital with the primary objective of comparing the incidence of CP before and after the implementation of this protocol. Among the secondary outcomes, we ought to highlight the incidence of cognitive deficits and necrotizing enterocolitis and the mortality in both groups. The sample consisted of preterm newborns delivered before 32 weeks of gestation in 2011−2012 (prior to the implementation of the protocol) and in 2016−2018 (after the implementation of the protocol, whose mothers had received magnesium sulfate for neuroprotection). The clinical and epidemiological characteristics of both groups were comparable.
ResultsWe collected data for a total of 523 patients, 263 and 260 in each group. As regards the primary outcome, we did not find statistically significant differences between groups. We observed a statistically significant reduction in mortality and the risk of severe necrotizing enterocolitis in the group of patients born in the 2016−2018 period and between 26+0 and 27+6 weeks of gestation, whose mothers had received magnesium sulfate.
ConclusionsIn our study, the administration of magnesium sulfate to mothers at risk of preterm birth did not decrease the risk of developing CP.
En 2016 se desarrolló en nuestro centro un protocolo de administración antenatal de sulfato de magnesio en gestantes con riesgo de parto pretérmino inminente como método para disminuir el riesgo de parálisis cerebral (PC).
Material y métodosSe realizó un estudio observacional y retrospectivo en un hospital de Nivel IIIC con objetivo principal de comparar la incidencia de PC previa y posteriormente a la puesta en marcha de este protocolo. Con respecto a los objetivos secundarios, a destacar la incidencia de déficit cognitivo, enterocolitis necrotizante y mortalidad en ambos grupos. Los pacientes incluidos fueron recién nacidos prematuros por debajo de 32 semanas de edad gestacional nacidos en los años 2011−2012 (previo a la instauración del protocolo) y 2016−2018 (posteriormente a la instauración del protocolo, cuyas madres habían recibido sulfato de magnesio como neuroprotector). Las características clínicas y epidemiológicas de ambos grupos fueron comparables entre sí.
ResultadosSe recogieron datos de un total de 523 pacientes, 263 y 260 de cada grupo. Con respecto al objetivo principal, no se encontraron diferencias estadísticamente significativas. Se objetivó, en el grupo de pacientes nacidos entre 2016−2018 y con edad gestacional entre 26 + 0 y 27 + 6 semanas, cuyas madres recibieron sulfato de magnesio, una reducción estadísticamente significativa de la mortalidad y del riesgo de enterocolitis necrotizante grave.
ConclusionesEn nuestro trabajo, el sulfato de magnesio administrado a madres en riesgo de parto prematuro, no disminuyó el riesgo de desarrollar PC.
Preterm birth is the most frequent cause of perinatal morbidity and mortality, accounting for 75% of neonatal deaths unrelated to congenital anomalies. Preterm newborns are also at increased risk of neurologic deficits, such as cerebral palsy (CP), blindness, deafness or cognitive impairment, understood as developmental delay or intellectual disability.
Cerebral palsy is the most frequent cause of motor impairment in children and the main cause of severe physical disability.1 The International Executive Committee for the definition of Cerebral Palsy proposed the following definition: “a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain. The motor disorders of cerebral palsy are often accompanied by disturbances of sensation, perception, cognition, communication, and behavior”.1 Its prevalence is estimated at approximately 2 in 1000 live births, and preterm birth is one of the main risk factors, with the risk increasing proportionally with decreasing gestational age.2
It is essential to consider the implementation of effective strategies to reduce the incidence of CP. One such strategy is the antenatal administration of magnesium sulfate (MgSO4), which acts on several pathways in the nervous system, preventing excitotoxicity and the activation of apoptotic pathways.3 Several randomised controlled trials have been published4–7 and later evaluated in a series of meta-analyses8–10 and Cochrane reviews.11–13 The main conclusions were that MgSO4 could reduce the risk of moderate to severe CP and improve neurodevelopmental outcomes in preterm infants, with no evidence of serious adverse events in the patients or their mothers, so this could be a valid intervention to offer all mothers at risk of preterm birth.
In light of the current evidence, in 2016 our hospital introduced a protocol for antenatal administration of MgSO4 to pregnant women at up to 32 weeks of gestation at risk of imminent preterm birth.
The primary objective of our study was to determine whether antenatal administration of MgSO4, per the hospital protocol, reduced the incidence of CP at 2 years of postmenstrual age, stratified by gestational age at birth.
The secondary objective was to determine whether antenatal administration of MgSO4 reduced the risk of cognitive impairment, the presence of features of moderate to severe brain injury in the transfontanellar ultrasound scan (intraventricular haemorrhage grade 3 and intracerebral haemorrhagic stroke), bronchopulmonary dysplasia, necrotising enterocolitis, retinopathy of prematurity and death.
Material and methodsWe conducted a single centre, retrospective observational and analytical study. The sample included preterm infants born before 32 weeks of gestation in our hospital in the 2011−2012 period (before the introduction of the MgSO4 protocol) and the 2016−2018 period (after the introduction of the protocol).
The inclusion and exclusion criteria were:
- none–
Inclusion criteria: preterm birth between 24 and 32 weeks of gestation, birth in 2011−2012 or 2016−2018.
- none–
Exclusion criteria: major congenital anomaly or prenatal diagnosis of chromosomal disorder.
- none–
Below are the definitions of key terms in this study:
- none-
Infantile cerebral palsy (ICP)
Permanent and nonprogressive motor disability secondary to an insult during the foetal/neonatal period resulting in impaired function and limitation of activity.
The severity was classified according to the Gross Motor Function Classification System, expanded and revised (GMFCS-E & R)14:
- I
(Mild): walks without limitations, with limitations for more advanced motor activities.
- II
(Moderate): walks without mobility devices, with limitations walking outdoors and in the community.
- III
(Moderate): walks with mobility devices. Limitations walking outside the home and in the community.
- IV
(Severe): self-mobility with limitations, transported or requiring mobility device outside the home and in the community.
- V
(Severe): severely limitations to self-mobility, even with adaptive equipment or assisted technology.
- none-
Cognitive impairment, defined as:
- •
A score of less than 85 on the validated Brunet-Lézine developmental scale, administered at 2 years of postmenstrual age in the department of child psychology.
- •
Delayed psychomotor development, evinced during the successive checkups by the primary care paediatrician though clinical interviews with the patient and the family.
- none-
Moderate-severe lesion in the transfontanellar ultrasound examination: for the study, we considered patients with intraventricular haemorrhage occupying more than 50% of the ventricular volume associated to acute ventricular distension (grade III haemorrhage) or evidence of haemorrhagic stroke in the periventricular white matter ipsilateral to intraventricular bleeding (periventricular haemorrhagic stroke).15
- none-
Necrotising enterocolitis (NEC): for the study, we considered patients with enterocolitis requiring surgical intervention (Bell stage IIIB).
- none-
bronchopulmonary dysplasia (BPD): for the study, we considered patients with moderate BPD (respiratory support with nasal prongs and a fraction of inspired oxygen [FiO2] > 0.21, but < 0.3 at 36 weeks of postmenstrual age) and severe BPD (respiratory support with nasal prongs and a FiO2 > 0.3 or with continuous positive airway pressure [CPAP]/non-invasive mechanical ventilation at 36 weeks of postmenstrual age).
- none-
Retinopathy of prematurity (ROP): for the study, we considered patients with ROP stage III or higher at the time of discharge from the neonatal unit (stage III: vascular proliferation into the cavity of the eye, stage IV: subtotal retinal detachment, stage V: total retinal detachment).
- none-
Full course of antenatal steroids: administration of 2 doses, at least 24 h apart and at least 24 h before birth.
- none-
Advanced life support (ALS): need of orotracheal intubation (ALS type 4) or of intravenous or intratracheal administration of adrenaline (ALS type V) at birth.
- none-
- none-
Chorioamnionitis:
- •
Suspected diagnosis: documented maternal fever (>39°C in any isolated measurement or ≥38°C sustained for more than 30 min) and at least one of the following: foetal tachycardia (>160 bpm for 10 min or longer), maternal leucocytosis (count >15,000 mm3) or purulent or foul amniotic fluid.
- •
Confirmed diagnosis: meets all previous criteria and evidence of infection in the histopathological examination and testing of the amniocentesis specimen (positive Gram stain or culture of amniotic fluid) or the placenta.16
With respect to the approach to the administration of MgSO4, we ought to highlight the variability between centres. The administered dose was based on studies conducted by Pritchard, who sought to establish the optimal dose for treatment of preeclampsia (1979),17 and the retrospective analysis of the data obtained in the Beneficial Effects of Antenatal Magnesium Sulfate (BEAM) trial.5
A study conducted by Alonso et al. established that the concentration of MgSO4 in the child was linearly proportional to the dose received by the mother and that, even the administration of the loading bolus alone, MgSO4 could be found in foetal blood, so the authors recommended administration of MgSO4 to all patients at risk of preterm birth, even if it was limited to the initial bolus.18
According to the protocol established in our centre, administration of MgSO4 is indicated in pregnant women between 23 and 31+6 weeks of gestation at risk of imminent birth or in whom termination of pregnancy within 24 h has been planned due to a maternal or foetal condition. The dosage consists in the intravenous administration of a loading bolus of 4.5 g over 20 min followed by a maintenance dose of 1 g per hour for 24 h or until delivery.
There is also controversy regarding the indication of retreatment if delivery ends up not taking place. The most recent recommendations published in UptoDate (December 2022) recommend against routine retreatment, as there is limited evidence in support of it in the literature.19
In the statistical analysis, we summarised continuous data as mean and standard deviation (SD) and categorical data as relative frequencies and percentages. In the case of quantitative data that did not follow a normal distribution, the data are expressed as median and interquartile range (IQR). We assessed the normality of the distribution with the Kolmogorov-Smirnov test.
We analysed the association of the administration of MgSO4 with other qualitative variables by means of the χ2 test or the Fisher exact test. To measure the strength of the association, we calculated odds ratios with the corresponding confidence intervals. The association between quantitative variables was analysed with the Pearson or Spearman correlation coefficients.
To compare the means of 2 or more groups, we used parametric tests (Student t or analysis of variance) or nonparametric tests (Mann-Whitney, Kruskal-Wallis) as applicable based on the shape of the distribution and the number of patients in each group.
The statistical analysis was conducted with the STATA software. We considered P values of less than 0.05 statistically significant.
ResultsWe collected data for a total of 523 patients born preterm in our hospital before 32 weeks of gestation. Of this total, 263 were born in the 2011−2012 period (pre-intervention group, before the introduction of the MgSO4 protocol) and 260 in the 2016−2018 period (post-intervention group, after the introduction of the MgSO4 protocol).
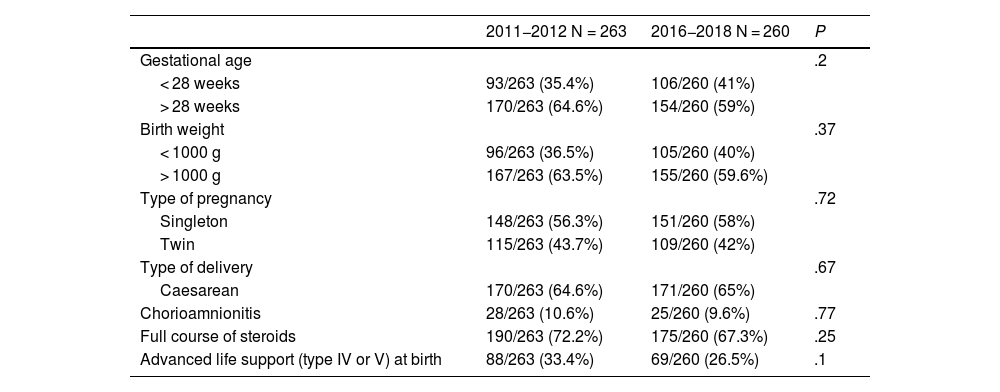
The groups were comparable from the outset of the study, as can be seen in Table 1. The sex distribution of the sample was 56% female and 46% male. The overall mortality was 17% (89 patients).
Comparison of the two groups.
| 2011−2012 N = 263 | 2016−2018 N = 260 | P | |
|---|---|---|---|
| Gestational age | .2 | ||
| < 28 weeks | 93/263 (35.4%) | 106/260 (41%) | |
| > 28 weeks | 170/263 (64.6%) | 154/260 (59%) | |
| Birth weight | .37 | ||
| < 1000 g | 96/263 (36.5%) | 105/260 (40%) | |
| > 1000 g | 167/263 (63.5%) | 155/260 (59.6%) | |
| Type of pregnancy | .72 | ||
| Singleton | 148/263 (56.3%) | 151/260 (58%) | |
| Twin | 115/263 (43.7%) | 109/260 (42%) | |
| Type of delivery | .67 | ||
| Caesarean | 170/263 (64.6%) | 171/260 (65%) | |
| Chorioamnionitis | 28/263 (10.6%) | 25/260 (9.6%) | .77 |
| Full course of steroids | 190/263 (72.2%) | 175/260 (67.3%) | .25 |
| Advanced life support (type IV or V) at birth | 88/263 (33.4%) | 69/260 (26.5%) | .1 |
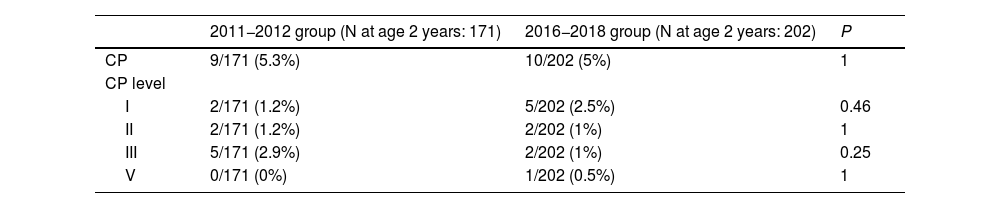
As regards the primary outcome, 19 patients (5%) had ICP based on the data available at age 2 years (N = 373). Of these patients, 37% had mild forms of CP (grade I) and 57% moderate forms (grades II and III). Only one patient had severe CP (grade V).
In the group of patients born in 2011−2012 (before the introduction of the antenatal MgSO4 protocol), 9 out of 171 had CP at age 2 years (5.3%) and in the group of patients born in 2016−2018 (after the introduction of the protocol) 10 out of 202 had CP at 2 years (5%). We did not find differences in the severity of CP between groups (Table 2).
Comparison of the incidence of cerebral palsy and its severity in the 2 groups.
| 2011−2012 group (N at age 2 years: 171) | 2016−2018 group (N at age 2 years: 202) | P | |
|---|---|---|---|
| CP | 9/171 (5.3%) | 10/202 (5%) | 1 |
| CP level | |||
| I | 2/171 (1.2%) | 5/202 (2.5%) | 0.46 |
| II | 2/171 (1.2%) | 2/202 (1%) | 1 |
| III | 5/171 (2.9%) | 2/202 (1%) | 0.25 |
| V | 0/171 (0%) | 1/202 (0.5%) | 1 |
CP, cerebral palsy.
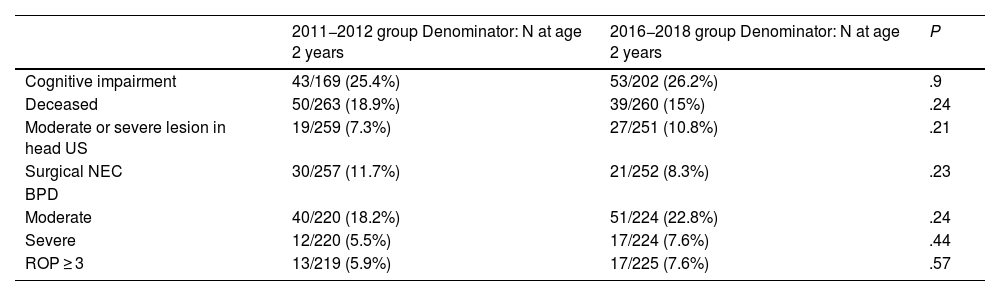
A total of 96 patients had cognitive impairment (25.8%) based on the data available at age 2 years (N = 371). It was found in 43 out of 169 patients (25.4%) in the group born in 2011−2012 and in 53 out of 202 patients (26.2%) in the group born in 2016−2018. Thus, we did not find statistically significant differences between the two groups. We also found no significant differences in mortality or any other secondary outcome (Table 3).
Comparison of other variables in the 2 groups.
| 2011−2012 group Denominator: N at age 2 years | 2016−2018 group Denominator: N at age 2 years | P | |
|---|---|---|---|
| Cognitive impairment | 43/169 (25.4%) | 53/202 (26.2%) | .9 |
| Deceased | 50/263 (18.9%) | 39/260 (15%) | .24 |
| Moderate or severe lesion in head US | 19/259 (7.3%) | 27/251 (10.8%) | .21 |
| Surgical NEC | 30/257 (11.7%) | 21/252 (8.3%) | .23 |
| BPD | |||
| Moderate | 40/220 (18.2%) | 51/224 (22.8%) | .24 |
| Severe | 12/220 (5.5%) | 17/224 (7.6%) | .44 |
| ROP ≥ 3 | 13/219 (5.9%) | 17/225 (7.6%) | .57 |
BPD, bronchopulmonary dysplasia; NEC, necrotising enterocolitis; ROP, retinopathy of prematurity; US, ultrasound.
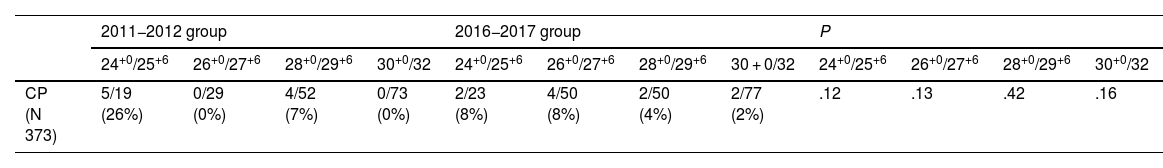
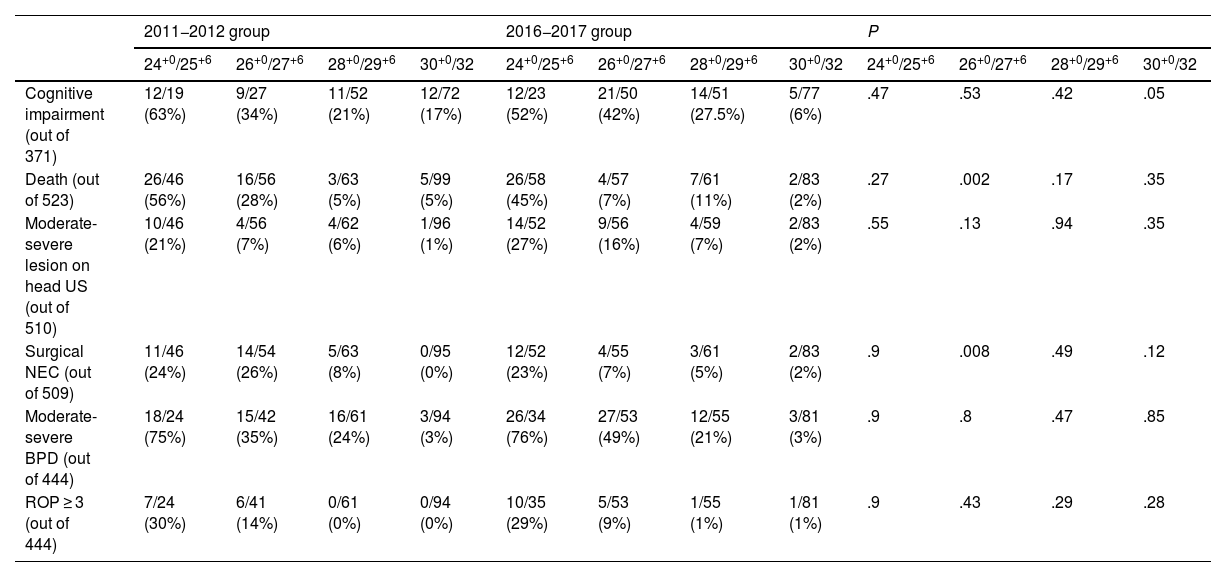
The analysis stratified by gestational age (in weeks) also found no significant differences between groups in the primary outcome, the main objective of our study (finding whether antenatal administration of MgSO4 decreases the risk of CP) (Table 4).
Comparison of the incidence of CP in the two groups in the analysis stratified by weeks of gestation.
| 2011−2012 group | 2016−2017 group | P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24+0/25+6 | 26+0/27+6 | 28+0/29+6 | 30+0/32 | 24+0/25+6 | 26+0/27+6 | 28+0/29+6 | 30 + 0/32 | 24+0/25+6 | 26+0/27+6 | 28+0/29+6 | 30+0/32 | |
| CP (N 373) | 5/19 (26%) | 0/29 (0%) | 4/52 (7%) | 0/73 (0%) | 2/23 (8%) | 4/50 (8%) | 2/50 (4%) | 2/77 (2%) | .12 | .13 | .42 | .16 |
CP, cerebral palsy.
When it came to the secondary outcomes, we found a statistically significant reduction in mortality in the patients born between 26+0 and 27+6 weeks of gestation who received antenatal MgSO4 (28% vs 7%; P 0.002), as well as a statistically significant decrease in the incidence of severe NEC (26% vs 7%; P 0.008).
In patients born between 30+0 to 32 weeks of gestational age, administration of MgSO4 was associated with a decreased risk of cognitive impairment (17% vs 6%; P 0.05) (Table 5).
Comparison of the incidence of cognitive impairment and other variables in the two groups in the analysis stratified by weeks of gestation.
| 2011−2012 group | 2016−2017 group | P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24+0/25+6 | 26+0/27+6 | 28+0/29+6 | 30+0/32 | 24+0/25+6 | 26+0/27+6 | 28+0/29+6 | 30+0/32 | 24+0/25+6 | 26+0/27+6 | 28+0/29+6 | 30+0/32 | |
| Cognitive impairment (out of 371) | 12/19 (63%) | 9/27 (34%) | 11/52 (21%) | 12/72 (17%) | 12/23 (52%) | 21/50 (42%) | 14/51 (27.5%) | 5/77 (6%) | .47 | .53 | .42 | .05 |
| Death (out of 523) | 26/46 (56%) | 16/56 (28%) | 3/63 (5%) | 5/99 (5%) | 26/58 (45%) | 4/57 (7%) | 7/61 (11%) | 2/83 (2%) | .27 | .002 | .17 | .35 |
| Moderate-severe lesion on head US (out of 510) | 10/46 (21%) | 4/56 (7%) | 4/62 (6%) | 1/96 (1%) | 14/52 (27%) | 9/56 (16%) | 4/59 (7%) | 2/83 (2%) | .55 | .13 | .94 | .35 |
| Surgical NEC (out of 509) | 11/46 (24%) | 14/54 (26%) | 5/63 (8%) | 0/95 (0%) | 12/52 (23%) | 4/55 (7%) | 3/61 (5%) | 2/83 (2%) | .9 | .008 | .49 | .12 |
| Moderate-severe BPD (out of 444) | 18/24 (75%) | 15/42 (35%) | 16/61 (24%) | 3/94 (3%) | 26/34 (76%) | 27/53 (49%) | 12/55 (21%) | 3/81 (3%) | .9 | .8 | .47 | .85 |
| ROP ≥ 3 (out of 444) | 7/24 (30%) | 6/41 (14%) | 0/61 (0%) | 0/94 (0%) | 10/35 (29%) | 5/53 (9%) | 1/55 (1%) | 1/81 (1%) | .9 | .43 | .29 | .28 |
BPD, bronchopulmonary dysplasia; NEC, necrotising enterocolitis; ROP, retinopathy of prematurity; US, ultrasound.
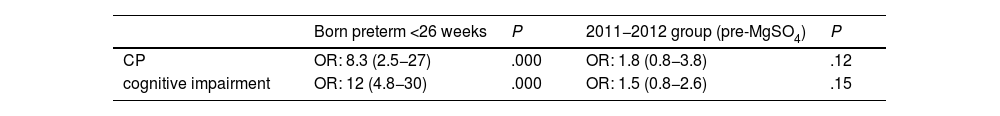
The multivariate analysis did not find significant differences in the incidence of CP or cognitive impairment between the two groups, while preterm birth was an independent risk factor for both (Table 6).
DiscussionThe main risk factor for CP is preterm birth, whose incidence increases with decreasing gestational age. It is estimated that CP is 70 times more frequent in children born before 28 weeks of gestation.1 A study conducted in France and published in 202120 found a prevalence of CP of 3.4% in preterm infants born before 33 weeks of gestation, a smaller proportion compared to our study (5%). However, to the proportion we found may be biased, as we were only able to obtain data at age 2 years for 373 out of the 523 patients included in the initial sample. Most of the patients whose data was not available were either from a different autonomous community in Spain or were not followed up in the public health system of our autonomous community, which precluded access to their electronic health records. If we calculated the proportion of patients with CP relative to the total sample (523 patients), it would be of 3.6%, similar to the one reported in France.
Our study did not find evidence of a reduction in the incidence of CP in preterm infants whose mothers had received antenatal treatment with antenatal con MgSO4, but such evidence has been found in the past. In the 1990s, Nelson et al. and Schendel et al. incidentally found a decrease in the frequency of CP in infants born to mothers who had received MgSO4 for treatment of preeclampsia or tocolysis.3,21 In this context, 3 clinical trials were carried out between 1996 and 2004 (ACTO MgSO4, PREMAG trial and BEAM trial),4–6 followed by 3 meta-analyses,8–10 which determined that the risk of CP decreased in infants exposed to MgSO4 in utero following antenatal administration of MgSO4(relative risk [RR], 0.7; 95% confidence interval, 0.55−0.89). Other authors have found a decrease in mortality in exposed patients18 and a decrease in the composite outcome of death and neurodevelopmental impairment in infants born before 29 weeks of gestation with a history of intrauterine growth restriction.22
It is hypothesised that MgSO4 can increase the secretion of brain-derived neurotrophic factors,23 which could promote cerebral maturation and resilience against insults associated with prematurity. It has also been associated with vascular tone and maintenance of systemic perfusion, reducing the rate of oxygen consumption in the brain. This was observed through the monitoring of regional cerebral oxygen saturation in a study conducted by Stark et al. in preterm infants delivered before 30 weeks of gestation, in whom exposure to MgSO4 was associated with a reduction in cerebral oxygen consumption in the first 24 h post birth.24 Magnesium sulfate also acts as a N-methyl-D-aspartate receptor antagonist, so it has been proposed that it may reduce excitotoxicity following a hypoxic-ischaemic insult.5,6,23
In our study, we found a statistically significant reduction in mortality (P = .002) in infants born between 26+0 and 27+6 weeks of gestation, which was consistent with the findings of Alonso et al.,18 and a lower severity of cognitive impairment (P = .05) in infants born between at 30+0 and 32 weeks of gestation who had received antenatal MgSO4. We did not find significant differences between the groups in the development of CP, ROP or any other neurologic complication, such as moderate to severe lesions in the transfontanellar ultrasound.
When it comes to adverse events, the three largest clinical trials4–7 and successive reviews, such as a meta-analysis published in 2016,10 have not found evidence of serious adverse effects, and the antenatal administration of MgSO4 is considered a safe practice for both mother and foetus. In fact, in our study the proportion of severe NEC was significantly lower (P = .008) in preterm infants whose mothers had received MgSO4 in the subset born between 26+0/27+6 weeks of gestation.
We believe that this reduction in mortality and the incidence of NEC in the group born preterm at 26+0/27+6 weeks of gestation in the 2016−2018 period could be explained by the changes and improvements in the management of these patients, among which the most important in our study was the antenatal administration of MgSO4 to the mothers. We consider this intervention to be more important in this subset of patients compared to the rest because, in infants born before 26 weeks of gestation, or extremely preterm, development and its complications are probably more affected by the degree of prematurity than by any treatment or improvement in management, while in the group born after 30 weeks of gestation, gestational age and maturity offer advantages in comparison to infants delivered at younger gestational ages.
Chief among the strengths of the study are its large sample of patients recruited in 2 time periods but with comparable clinical and epidemiological characteristics, not only in respect of the perinatal data but also in the presentation of diseases commonly associated with prematurity, such as NEC, BPD, brain damage and ROP, and the collection of data at birth and at 2 years of postmenstrual age.
Chief among its limitations, since it was a retrospective study, was the indirect collection of data, as they were retrieved from the electronic health records of the patients. We must also highlight the missing data due to the patients lost to follow-up at the 2-year timepoint, which may have reduced the statistical power of the findings. In the group born in the 2011−2012 period (before the introduction of the protocol), we estimate that a small proportion of infants had received antenatal MgSO4. Based on the information documented in the maternal electronic health records, 19 of the 263 (7.2%) patients had received MgSO4, in 14 cases for treatment of maternal eclampsia/preeclampsia and in the rest for neuroprotection. Given this situation, and since the dosage of MgSO4 had yet to be standardised in the protocol currently implemented, we did not take these cases into account when we counted the overall number of patients who received MgSO4 and we included them in the preintervention group. We also ought to mention that since the groups correspond to different time windows, even though the difference is of only a few years, there may have been changes in management protocols, clinical guidelines and other aspects that may have biased the results.
In conclusion, our study found no differences between groups in the development of CP. The fact that this is such a widely used treatment underscores the need to continue investigating this practice.
Conflicts of interestThe authors have no conflicts of interest to declare.
Previous meeting: this study was presented at the Congress of the Sociedad Española de Neonatología, October 25–29, 2021.