The importance of immunization was underscored anew by the SARS-CoV-2 pandemic, which stopped being a global emergency with vaccination. Fortunately, today we have new tools against another virus, respiratory syncytial virus (RSV): a monoclonal antibody (MAb), nirsevimab, and a vaccine (RSVPreF vaccine against the RSV prefusion F protein).
The Asociación Española de Pediatría (Spanish Association of Paediatrics) was the first scientific society in the world to recommend the inclusion of nirsevimab in the routine immunization schedule for newborns and infants aged less than 6 months, with annual administration in children aged less than 2 years with underlying disease increasing the risk of severe RSV infection, as contemplated in the official immunization schedule published on January 1, 2023.1 The reasons for its inclusion in the schedule were the efficacy and safety data from clinical trials, the high burden of RSV-associated disease and the authorisation of nirsevimab by the European Commission on October 31, 2022 following the recommendation of the European Medicines Agency (EMA). Nirsevimab acts by binding an epitope in antigenic site Ø on the prefusion F protein (pre-F) of RSV, blocking the fusion of the virus with the cells of the tracheobronchial tree. Compared to palivizumab, nirsevimab offers the advantage that its neutralising potency is 50- to 100-fold greater, and it also has a longer half-life, so that a single dose can confer protection for the entire RSV season.
The current issue of Anales de Pediatría includes a special article presenting the position statement of the Sociedad Española de Infectología Pediátrica2 (SEIP, Spanish Society of Paediatric Infectious Diseases) and a scientific letter written by the Sociedad Española de Neonatología3 (SENeo, Spanish Society of Neonatology) detailing their respective recommendations for the use of nirsevimab in the upcoming RSV season.
The article by the SEIP2 presents a critical review of the literature conducted with the Delphi and GRADE methodologies with the purpose of developing recommendations for the use of nirsevimab to reduce the incidence of RSV-associated bronchiolitis, lower respiratory tract infection and hospitalization, and issuing strong recommendations for its use in infants born during the RSV season and those aged less than 6 months entering their first season, as well as for infants born preterm between 29 and 35 weeks of gestation, haemodynamically significant heart disease or chronic pulmonary disease, and weak recommendations for its use in the general population of healthy term and late preterm infants on account of the lack of additional published studies reproducing the findings of the clinical trials and the existence of uncertainties regarding aspects like the implementation of its administration, its cost, acceptance by families, post-authorization safety following its large-scale distribution or the development of resistance by the virus. We consider that the strength of the recommendation would have probably passed from weak to strong following the presentation of the findings of the Hospitalized RSV Monoclonal Antibody Prevention (HARMONIE) trial, presented in May 2023 at the 41th Meeting of the European Society for Paediatric Infectious Diseases (ESPID),4 although since these data were not published at the time, they could not be taken into account.
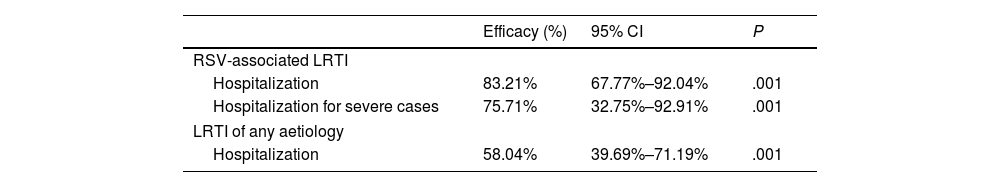
The HARMONIE trial was a phase IIIb trial conducted across nearly 250 sites in France, Germany and the United Kingdom (UK) during the 2022–2023 RSV season that analysed the use of nirsevimab under real-world conditions. Its primary objective was to assess the efficacy and safety of nirsevimab compared to no intervention (standard of care) for prevention of hospitalization due to RSV-associated lower respiratory tract infection (LRTI) in infants aged less than 12 months born to term or preterm at or after 29 weeks of gestation who were not eligible for immunization with palivizumab, and its secondary objective to assess its efficacy and safety for prevention of hospitalization due to severe RSV-associated LRTI, defined as an oxygen saturation of less than 90% and need of supplemental oxygen, and hospitalization due to LRTI of any aetiology. The duration of adverse events monitoring was set to 12 months. Table 1 presents the preliminary data on the efficacy of nirsevimab.4
Results of the HARMONIE trial in 8050 infants aged less than 12 months. 4037 received nirsevimab and 4021 the standard of care. Efficacy data at 180 days after its administration.
| Efficacy (%) | 95% CI | P | |
|---|---|---|---|
| RSV-associated LRTI | |||
| Hospitalization | 83.21% | 67.77%–92.04% | .001 |
| Hospitalization for severe cases | 75.71% | 32.75%–92.91% | .001 |
| LRTI of any aetiology | |||
| Hospitalization | 58.04% | 39.69%–71.19% | .001 |
CI, confidence interval; LRTI, lower respiratory tract infection; RSV, respiratory syncytial virus.
The safety profile has been found to be favourable and consistent with the findings of confirmatory studies. With these results, it is reasonable to infer that the burden on health care systems and associated costs would be significantly reduced if all infants received nirsevimab.
A new analysis of the MELODY trial was also presented at the 2023 ESPID meeting, which had found a similar incidence of infection by respiratory viruses other than RSV in participants given nirsevimab compared to those who received placebo, suggesting that there is no replacement of RSV by other viruses after administration of the monoclonal antibody.
Nirsevimab has also been authorised by other regulatory agencies, for instance the Medicines and Healthcare products Regulatory Agency of the UK (7/11/2022), Health Canada (19/04/2023) and the Food and Drug Administration (FDA) of the United States (17/07/2023). In Spain, the guidelines for the use of nirsevimab against RSV for the 2023–2024 season,5 published on July 25, 2023, recommended its use in children at high risk of severe RSV infection and in infants aged less than 6 months during or at the beginning of the season (October-March). Several autonomous communities in Spain have already announced the introduction of this immunization for the upcoming season.
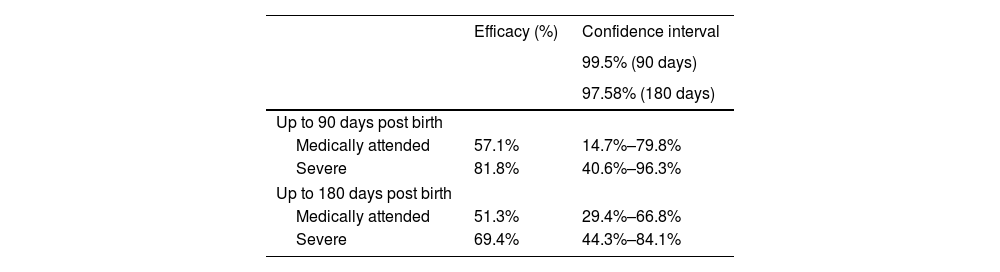
In August, the RSVPreF vaccine, a bivalent recombinant F protein subunit vaccine, was authorised for administration to pregnant women between 24 and 36 weeks of gestation (EMA) or between 32 and 36 weeks (FDA) for prevention of LRTI in infants up to age 6 months. This strategy seeks the passive immunization of the infant (through the passage of maternal antibodies to the foetus) through the active immunization (vaccination) of the mother. Its authorization was based on the results of the MATISSE phase III trial,6 presented in Table 2.6
MATISSE trial in 7358 healthy pregnant women aged 18 to 49 years, of who 3682 received RSVPreF and 3676 placebo. Efficacy of the vaccine in preventing RSV-associated lower respiratory tract infection in infants.
| Efficacy (%) | Confidence interval | |
|---|---|---|
| 99.5% (90 days) | ||
| 97.58% (180 days) | ||
| Up to 90 days post birth | ||
| Medically attended | 57.1% | 14.7%–79.8% |
| Severe | 81.8% | 40.6%–96.3% |
| Up to 180 days post birth | ||
| Medically attended | 51.3% | 29.4%–66.8% |
| Severe | 69.4% | 44.3%–84.1% |
RSV, respiratory syncytial virus; RSVPreF, RSV prefusion F vaccine.
No safety signals were detected in in maternal participants or in infants and toddlers up to 24 months of age. The incidence of adverse events reported within 1 month after injection or within 1 month after birth were similar in the vaccine group (13.8% of women and 37.1% of infants) and the placebo group (13.1% and 34.5%, respectively) The incidence of adverse events reported 1 month. There was a slight, although not statistically significant, increase in the incidence of preterm birth (5.6% in the vaccine group vs 4.7% in the placebo group).
The competent public health authorities will have to decide which strategy to implement taking into account the multiple determinants that may influence its success in terms of vaccination coverage, as done by the Joint Committee on Vaccination and Immunisation of the United Kingdom.7
In the future, an additional MAb, clesrovimab, will be available; it acts on antigenic site IV, present in both the prefusion- and postfusion configurations of RSV. It has a long half-life and its neutralising potency is similar to that of nirsevimab. Two randomised clinical trials are underway: one phase IIb/III trial comparing it to placebo in infants born preterm between 29 and 34 weeks of gestation, late preterm between 35–37 weeks and born to term, from which data are expected to be available in August 2024, and one phase III trial in patients at risk of severe infection by RSV with palivizumab as control, expected to be completed in April 2026.
The delay is greater for the trials of vaccines for children (phase II) of different types: intranasal live attenuated vaccines for children aged 6–24 meses, and recombinant viral vector-based vaccines, such as those with the adenovirus 26 vector, for children aged 12–24 months.
As paediatricians, we have high hopes that the now authorised pharmaceuticals for immunization against RSV will contribute to significantly reduce the important burden of disease the virus generates each season, especially in infants under 3 months, given that the current guidelines for the management of bronchiolitis consist merely of supportive care, leaving paediatricians with little recourse to modify the course of disease.
FundingNo external funding was received to carry out this project (analysis of published data, discussion, consensus and publication) beyond the logistical support provided by the AEP.
Conflicts of interestFJAG has participated in educational activities sponsored by Alter, Astra, GlaxoSmithKline, MSD, Pfizer and Sanofi and as a consultant in GlaxoSmithKline, MSD, Pfizer and Sanofi advisory boards.
AIA has participated in educational activities sponsored by GlaxoSmithKline, MSD and Pfizer and as a consultant in GlaxoSmithKline advisory boards, in addition to receiving funding from GlaxoSmithKline, MSD and Pfizer to attend educational activities in Spain.