Off-label drug prescribing is appropriate, necessary in many instances and frequent in pediatric care,1 although not free of risk. Due to the scarcity of pediatric clinical trials and the low frequency with which summaries of product characteristics (SmPCs) are updated, off-label use is part of everyday clinical practice. Globally, the described frequency of off-label prescribing at the primary care (PC) level varies widely (1.2%–62%2; 9%–52%3). In Spain, the prevalence of off-label prescribing (of any medicinal product) ranges between 27% and 51%.
Our objective was to determine the characteristics of off-label and unlicensed (UL) medicine prescribing at the primary care level in one health care area in Spain.
We conducted an observational study of the medicines prescribed in six pediatric caseloads (corresponding to three supervised medical intern-residents [MIRs] in pediatrics and three family physicians) of the Alcázar de San Juan health care area (16 PC clinics). The design was retrospective to avoid the Hawthorne effect. We included patients aged 0–14 years that received prescriptions in June and/or December 2018. On-label prescribing was defined as prescription of the medicine adhering to the indication, dose, schedule, patient age/weight range and route of administration specified in the SmPC; off-label prescribing as prescribing of a licensed drug that did not adhere to the SmPC in at least one of those variables; UL prescribing referred to the use of medicines that were not licensed, were contraindicated or were administered after modifying the dosage form in any way.
We classified the drugs according to the Anatomical Therapeutic Chemical Classification System (ATC). We obtained the consent of the parents/legal guardians to access the health records, as well as the approval of the Committee of Ethics, Research and Medicines of the health care area, in adherence to Royal Decree 957/2020 of November 3 regulating observational studies involving the use of medication in humans.
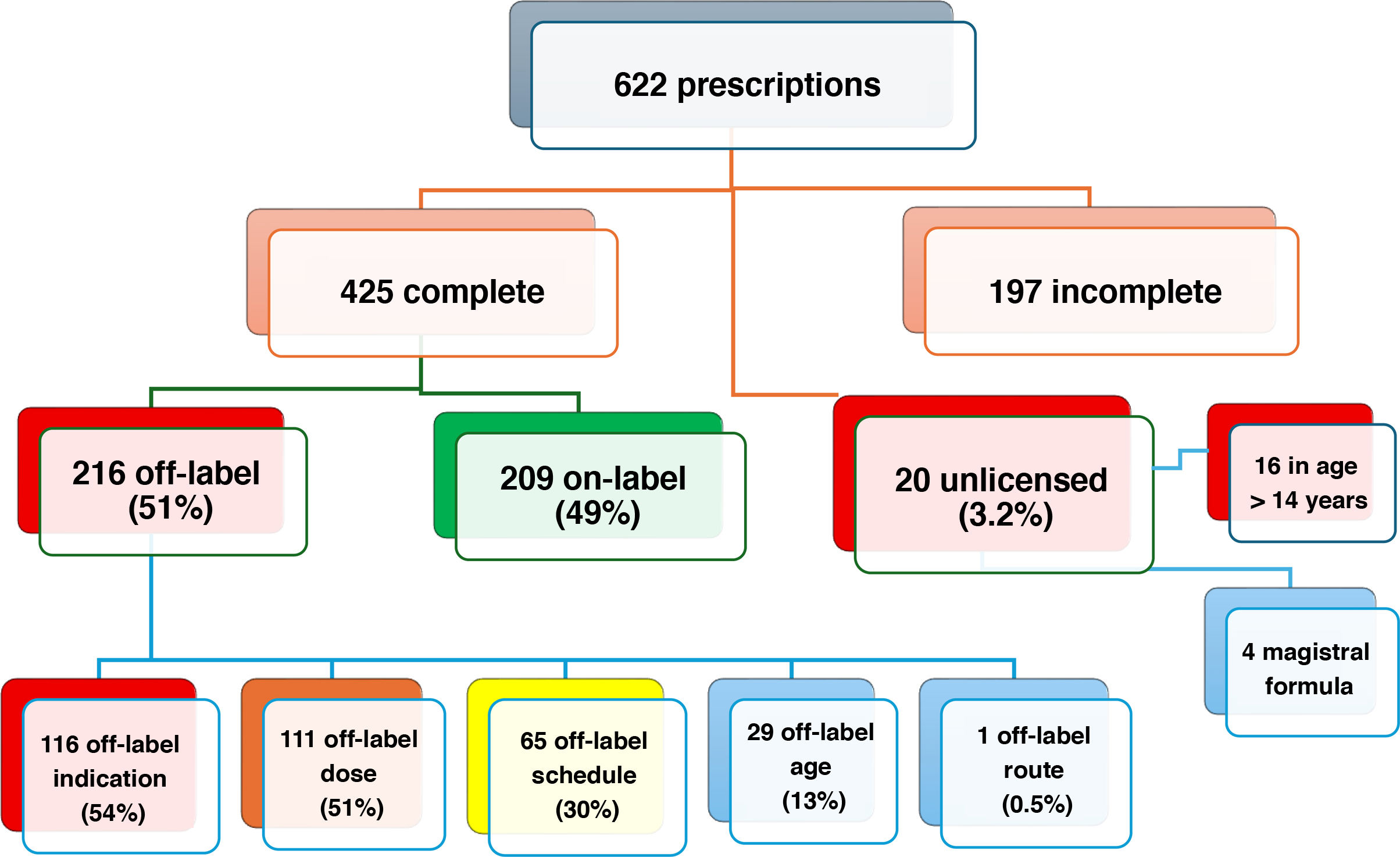
We obtained data for a total of 622 prescriptions in 418 patients. They corresponded to 192 different drugs and 82 active principles, with 1.4 medicines prescribed per patient. The mean age was 8.6 years (4 days-14.4 years). Children aged 6 or more years accounted for 65.5% of prescriptions.
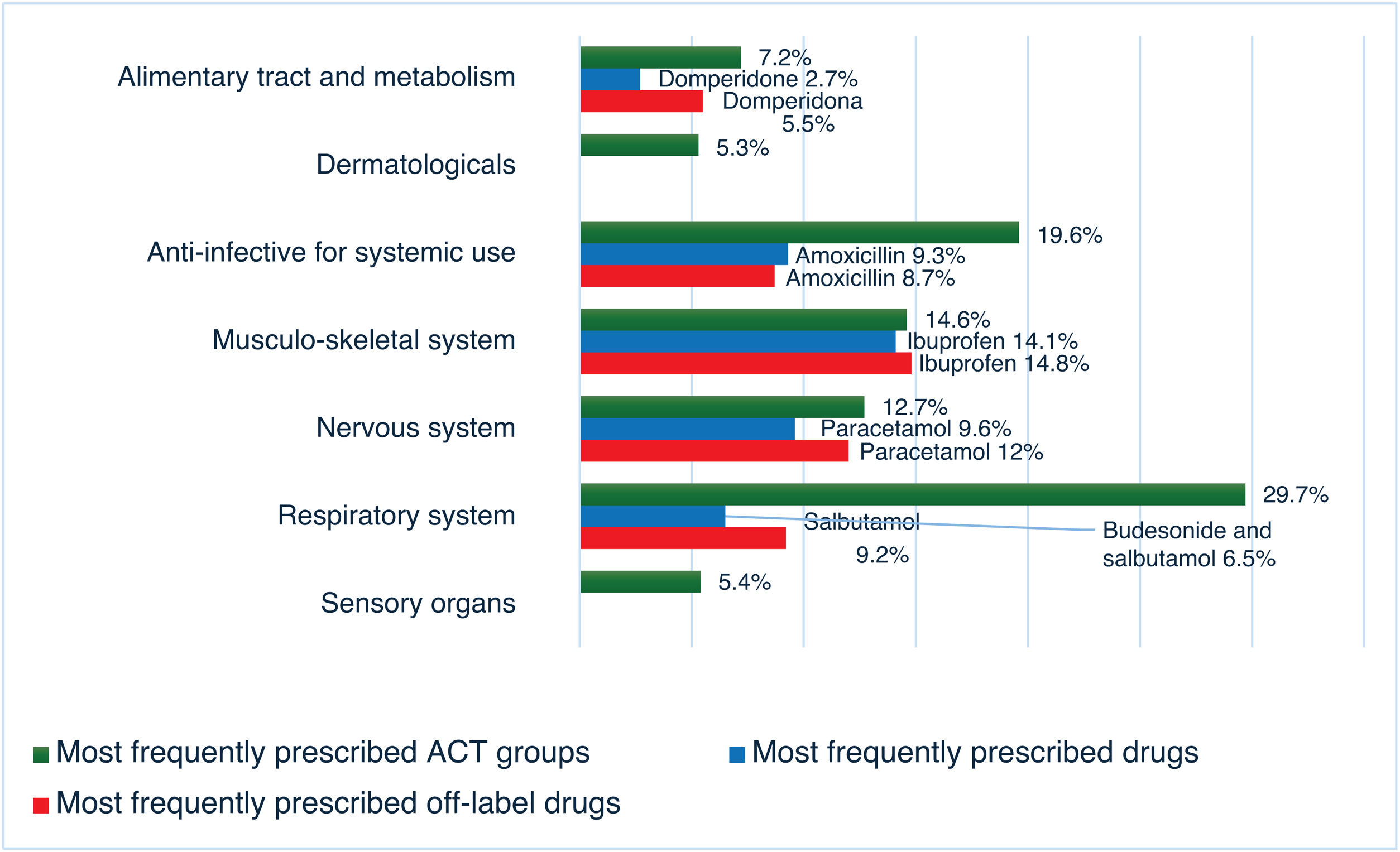
The variables required for the analysis were documented in 425 prescriptions. Fig. 1 shows the percentages of off-label and unlicensed prescriptions and the reasons for the categorization. The drugs most frequently prescribed off-label were ibuprofen (14.8%), paracetamol (12%), salbutamol metered-dose inhaler (MDI) (9.3%), amoxicillin (8.8%), cetirizine (6.9%), budesonide MDI (6%) and domperidone (5.5%).
Fig. 2 shows the most frequently prescribed drug groups and drugs, as well as the drugs most frequently prescribed off-label.
There were no significant differences in off-label prescribing based on the season.
In the multivariate analysis, the factors identified to contribute the most to off-label prescribing were: Respiratory System group, risk factor (RF) 11.2 (95% CI, 3–41.9), P < .0001; Alimentary Tract and Metabolism group, RF 7.3 (95% CI, 1.7–31.7), P = .008; Musculo-Skeletal System group, RF 6.4 (95% CI, 1.6–25.2), P = .008; Nervous System group, RF 5.7 (95% CI, 1.4–22.85), P = .014; physician not a pediatric specialist, RF 3.8 (95% CI, 2.3–6.15), P < .0001; patient age (for each additional year) RF 0.8 (95% CI, 0.8−0.99), P < .0001.
Further information on off-label prescribing is shown in Appendix B, supplementary Figs S1 and S2.
The rationale for using the drug had not been documented in any case, nor had the written or verbal informed consent of the parents or guardians.
Off-label drug prescribing continues to be very prevalent, it is an issue at the global level whose frequency increases with younger patient age, carries an increased risk of side effects and has barely improved despite regulatory efforts by health care authorities. The Committee on Medicines of the Asociación Española de Pediatría (Spanish Association of Pediatrics) highlighted its importance in 2021.1 Due to the heterogeneity in the definitions of off-label and unlicensed use, study methodology and data sources, the reported results vary.1 Many studies conducted at the PC level are based on large pharmaceutical registers, but did not analyze the indication or dosage, thereby underestimating the true frequency of off-label prescribing. In a review conducted by Balan et al.,2 only half the studies included all the necessary variables to adequately define off-label prescribing.
We found a proportion of off-label use of 51%, which is high, similar to the findings reported by Morales-Carpí et al. for PC in 20104 and other studies in Europe.2,3 Lower proportions have been reported in a study published in 2014 by Blanco-Reina et al. found a lower proportion (27%), although it was limited to urban settings (2 primary care centers and one hospital emergency department)5 and other studies at the PC level in our region.2,3
The frequency of unlicensed prescribing (3.2%) was substantially inferior compared to the study by Morales Carpí (12%),4 similar to those reported in other European studies2,3 and higher compared to the study by Blanco-Reina et al. (1%).5
The most frequent reason for categorization as off-label was not adhering to the established indications or dosage, in agreement with most previous studies.2–4,6 The drugs most frequently prescribed under off-label conditions belong to the respiratory system, anti-infective for systemic use, nervous system and alimentary tract and metabolism groups, which was similar to the previous literature.2,3
In conclusion, we found a high percentage of off-label prescriptions with no documentation of the rationale for their use or of parental consent. We must inform of the off-label use of any medicine, always supported by scientific evidence, and document it in the health record after obtaining parental consent.
FundingThis research did not receive any external funding.
The authors have no conflicts of interest to declare.
We thank Ms Beatriz Navarro Bravo and Dr Ángel Árias Árias for the statistical analysis. We also thank Dr Ramón Garrido Palomo and Dr Juan Trigo Moreno for their critical review of the article.
Previous meeting: this study was presented at the XIV Scientific Meeting of the Asociación de Pediatría de Atención Primaria de Castilla La-Mancha, held online on September 26, 2020.