Due to the significant increase in the number of cases of hand, foot and mouth disease (HFMD) among pre-school children during late 2011 and early 2012, a study has been proposed with the aim of describing the HFMD outbreak and analyzing the risk factors associated with suffering onychomadesis.
Patients and methodsA descriptive and analytical case–control study was designed. The study population was 376 children between 6 and 36 months old, living in the Basic Health area of Peligros (Granada). The study included an epidemiological survey of 28 cases and paired controls in order to collect data on the time, person and place, and implementing preventive actions and family health education. Finally a microbiological viral study of stool samples was made.
Results64% were girls with average age of 20.8 months. The clinical signs found were fever (75%), vesicular palmar eruption (71%), plantar eruption (68%), erosive stomatitis (64%), and nail loss (46%). The risk of getting sick was 14 times greater for those children attending a childcare centre and had contact with sick cases (OR 13.8; 95% CI; 3.79–50.18). The average time since onset of symptoms and onychomadesis was 52 days, and its appearance was linked to the presence of ulcers in the mouth (P=.006). Five samples were positive for enteroviruses Coxsackie A16.
ConclusionThere was an outbreak of HFMD detected by paediatricians and families. The cases presented with marked clinical symptoms, and the nail loss (onychomadesis) generated a social alarm. The cause of the outbreak was an enterovirus Coxsackie A16 transmitted among sick cases and through childcare centres.
Frente al aumento del número de casos de enfermedad boca-mano-pie (EBMP) entre la población preescolar a finales del 2011 y principios del 2012 y la presencia de onicomadesis, se planteó como objetivo describir el brote epidémico y analizar los factores de riesgo de enfermar y de presentar onicomadesis.
Pacientes y métodosSe diseñó un estudio descriptivo y analítico caso-control. La población de estudio fue de 376 niños entre 6 y 36 meses adscritos a la zona básica de salud de Peligros (Granada). Intervenciones: encuesta epidemiológica a 28 casos y controles, recogiendo variables de persona, lugar y tiempo; toma de medias preventivas y educación sanitaria. Estudio microbiológico viral de muestras de heces.
ResultadosEl 64% fueron niñas con edad media de 20,8 meses. La clínica fue de fiebre (75%), lesiones vesiculares en manos (71%), pies (68%), boca (64%) y caída de uñas (46%). El riesgo de enfermar fue de 14 veces más en aquellos que acudían a guardería y tuvieron contacto con enfermos (odds ratio ajustada 13,8; IC del 95%, 3,79–50,18). El tiempo medio desde inicio de síntomas y la onicomadesis fue de 52 días y su presencia estaba asociada a la presencia de úlceras en boca (p=0,006). Cinco muestras fueron positivas a enterovirus Coxsackie A16.
ConclusiónExistió un brote de EBMP detectado por los propios pediatras y familiares con una clínica llamativa y presencia de onicomadesis, que fue la que generó la alarma social. La causa del brote fue un enterovirus Coxsackie A16 transmitido entre casos conocidos con la enfermedad y en guarderías.
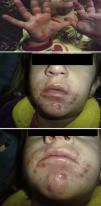
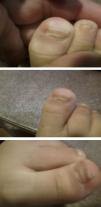
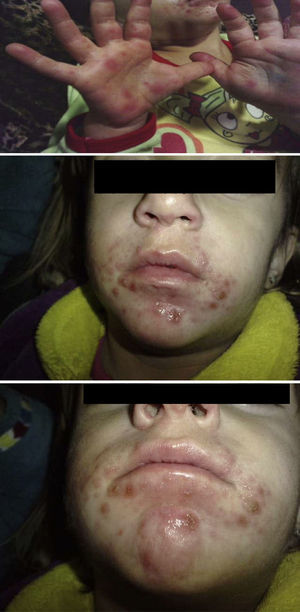
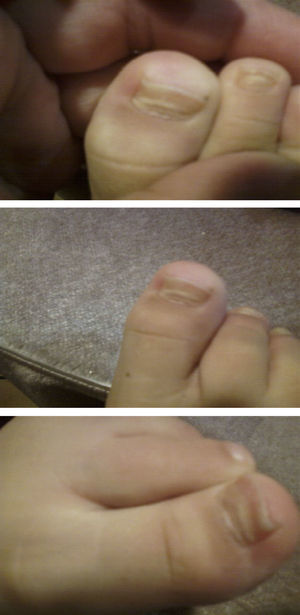
Hand, foot and mouth disease (HFMD) starts with a fever and about 2 days later numerous lesions appear in the mouth and tongue. Afterward, these lesions develop on hands and feet as small vesicles of approximately 3–7mm in diameter. These symptoms can be accompanied by general malaise, poor appetite, sore throat, cold symptoms, cough, diarrhoea, vomiting and adenopathies. The fever usually lasts 3–4 days; the mouth sores about 7 days; and the lesions on palms and soles about 10 days. If the vesicles in the mouth rupture, they can give rise to painful sores similar to aphthae. The patient may have difficulty eating if the lesions are plentiful, large, or depending on their location. The disease is benign and its complications rare, the most common being the shedding of the nails of the fingers and toes, especially in children, between 4 and 8 weeks after the onset of symptoms.1,2 Onychomadesis is the painless and inflammation-free separation or shedding of the nail plate from the nail bed beginning at its proximal end, with a new nail plate developing underneath (Figs. 1 and 2).
The disease is caused by viruses in the Enteroviridae family, with the highest prevalence corresponding to Coxsackievirus A16 (the most frequent cause) and Enterovirus 71 (which causes the highest morbidity and mortality).3–5 The aetiological agent cannot be identified in many outbreaks, and is simply established based on dermatologic and epidemiological characteristics.
The disease usually appears in outbreaks during the summer or autumn, affecting young children of 6 months to 4 years of age. The incubation period lasts 4–6 days. Its epidemic nature results from the ease with which enteroviruses are transmitted from person to person through direct contact, the air, and especially through the faecal-oral route.4–6 The individuals that contract the disease develop immunity to the specific virus that caused the infection.7
Transmission prevention is complicated by the large number of asymptomatic infections and the biological characteristics of enteroviruses, as infected patients secrete viral particles in their stool for weeks.8
Hand, foot and mouth disease, and then onychomadesis as one of its complications, was first described in 2000 in 5 children in Chicago (United States).9 In 2001, a similar report was published about 4 children in Europe.10 Cases from an onychomadesis outbreak were also described in Finland in 2008.11 Since 2008, several outbreaks of HFMD followed by onychomadesis have been reported in various Spanish locations: Zaragoza12, Valencia13, Mallorca14, A Coruña15 and Valladolid.16 The 2008 outbreak in Valencia clearly established an association between HFMD and onychomadesis.13,17
In February 2012, the paediatricians of the basic health area (BHA) of Peligros (Granada) reported a state of public alarm generated by the loss of nails in some preschool children following a cluster of HFMD cases that occurred in October and November of 2011. This was notified through the Red de Alerta de Salud Pública de Andalucía (Public Health Alert Network of Andalusia). A document was released describing measures to prevent new cases. A retrospective search of cases was performed, and data was collected on incident cases when patients sought care for this reason.
In this framework, we proposed two hypotheses: an increase in the incidence of HFMD, and the development of post viral onychomadesis in the group of patients in the BHA that attended a childcare centre.
Consequently, our aim was to describe the outbreak of HFMD in the BHA of Peligros between September 2011 and February 2012 and to analyse the risk factors for contracting the disease and for developing onychomadesis as a complication.
Patients and methodsThe study area consisted of the towns of Peligros and Pulianas in Granada. The population under study were the children of 6 months to 3 years of age assigned to the primary care clinical management unit (Unidad de Gestión Clínica [UGC]) of Peligros during the study period. The number of patients obtained from the Andalusia user database for this age range was 376.
We designed a case–control study to analyse the risk factors for acquiring the disease. We used the χ2 test, Student's t test, and multivariate logistic regression analysis, calculating the crude odds ratio (cOR), the adjusted odds ratio (aOR) and the 95% confidence interval. We performed the statistical analysis with the R software, version 2.12.1. The analysis of nail loss used the data of the cases that presented it and the cases that did not.
We defined a suspected case of HFMD as a child between 6 months and 3 years of age presenting with some of these signs and symptoms: fever, sore throat, upper respiratory tract infection and loss of appetite; with the physical examination revealing some of the following signs: vesicular eruption on hands, feet, nappy area or mouth accompanied by ulcers (ulcerated vesicles) in the throat and oral cavity. Thus, the cases in our study were those patients that fit the definition of a HFMD case whose parents sought care for those signs and symptoms at the paediatrics department of the UGC during the period under study. We selected a total of 28 cases. A control was selected for each case.
We identified cases from the electronic medical records (EMRs) of the Diraya information system available to healthcare providers in the Andalusia public health system. The controls were pair-matched by age and physical proximity to the home of the case and the childcare centres. We did this using Diraya, the listing of children included in the Plan Andaluz de Vacunación (vaccination plan of Andalusia) and Google maps.
The selection of controls and the inclusion of their data for the study variables were done in March 2012. For controls, we also collected the data corresponding to the epidemic season under consideration.
We developed an epidemiology survey to gather information. Before conducting the survey we did a pilot study, administering the questionnaire to the first cases and controls, and modified some items to make the questionnaire easier to understand. The variables for which data was collected were location (town and childcare centre), time (date of onset of symptoms, date of end of symptoms, date of the first related medical visit, date when onychomadesis started) and personal characteristics (age, sex, number of siblings, number of sick siblings, previous contact with known cases, visit to the paediatrician between September 2011 and February 2012). We collected data on the presence of fever, pharyngitis, upper respiratory tract infection, loss of appetite, throat ulcers, vesicles in hands, mouth, feet and nappy area, and ungual lesions. The survey was conducted by telephone, and the clinical data were collected from the EMRs.
For the last cases identified, 9 stool samples had been sent to the reference laboratory in Andalusia for virology testing, the microbiology laboratory of the Hospital Universitario Virgen de las Nieves of Granada, to identify the causative agent. The samples were resuspended in minimal essential medium Eagle and centrifuged at 2000rpm for 10min at 4°C. The supernatant was collected for culture and reverse transcription polymerase chain reaction (RT-PCR). Four 200μl supernatant aliquots were processed in parallel: one for RT-PCR and the other 3 for culture.
Following incubation, we tested the culture supernatant by RT-PCR to detect enterovirus growth in tubes with no observed cytopathic effect. To determine the enterovirus serotype from the samples and/or isolates, we performed nested RT-PCR with 5′ noncoding region primers. The products of this RT-PCR assay were subjected to bidirectional sequencing. We analysed the resulting sequences by sequence searching, alignment and comparison with the sequences available in the GenBank database using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and MEGA5.
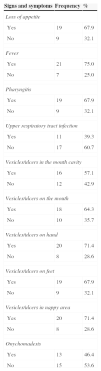
ResultsTable 1 describes the salient clinical features, with fever as the main symptom (75%), preceding the formation of vesicles and not exceeding 38°C. Vesicles presented in greater percentages on the hand, nappy area and foot than in the mouth and in the oral cavity. Figs. 1 and 2 show their presentation. The mean±standard deviation of disease duration was 6.14±3.84 days, with a minimum of 1 and a maximum of 20 days, excluding episodes of nail loss. The clinical features of onychomadesis were observed on the nails of hands and feet. It was painless and without inflammation of the nail matrix in the proximal region. One patient lost the whole nail. Beau's lines, transversal ridges and grooves on the nail plate that go from one lateral fold to another, develop as a result of the temporary cessation of nail formation.
Clinical features of hand, foot and mouth disease.
| Signs and symptoms | Frequency | % |
|---|---|---|
| Loss of appetite | ||
| Yes | 19 | 67.9 |
| No | 9 | 32.1 |
| Fever | ||
| Yes | 21 | 75.0 |
| No | 7 | 25.0 |
| Pharyngitis | ||
| Yes | 19 | 67.9 |
| No | 9 | 32.1 |
| Upper respiratory tract infection | ||
| Yes | 11 | 39.3 |
| No | 17 | 60.7 |
| Vesicles/ulcers in the mouth cavity | ||
| Yes | 16 | 57.1 |
| No | 12 | 42.9 |
| Vesicles/ulcers on the mouth | ||
| Yes | 18 | 64.3 |
| No | 10 | 35.7 |
| Vesicles/ulcers on hand | ||
| Yes | 20 | 71.4 |
| No | 8 | 28.6 |
| Vesicles/ulcers on feet | ||
| Yes | 19 | 67.9 |
| No | 9 | 32.1 |
| Vesicles/ulcers in nappy area | ||
| Yes | 20 | 71.4 |
| No | 8 | 28.6 |
| Onychomadesis | ||
| Yes | 13 | 46.4 |
| No | 15 | 53.6 |
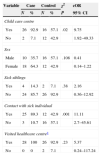
Table 2 analyses the risk factors under consideration. We found statistically significant differences between children that attended childcare and children that did not (cOR, 9.75; 95% CI, 1.92–49.33). We did not observe a higher risk of contracting disease in the presence of household cases of HFMD. The risk of contracting the disease was 11 times higher when there had been contact with a known case (cOR, 11.11; 95% CI, 2.7–45.61). We did not find significant differences by age (P=.57) or number of siblings (P=.35).
Analysis of studied risk factors for contracting hand, foot mouth disease.
| Variable | Case | Control | χ2 | cOR | ||
|---|---|---|---|---|---|---|
| N | % | N | % | P | 95% CI | |
| Child care centre | ||||||
| Yes | 26 | 92.9 | 16 | 57.1 | .02 | 9.75 |
| No | 2 | 7.1 | 12 | 42.9 | 1.92–49.33 | |
| Sex | ||||||
| Male | 10 | 35.7 | 16 | 57.1 | .108 | 0.41 |
| Female | 18 | 64.3 | 12 | 42.9 | 0.14–1.22 | |
| Sick siblings | ||||||
| Yes | 4 | 14.3 | 2 | 7.1 | .38 | 2.16 |
| No | 24 | 85.7 | 26 | 92.9 | 0.36–12.92 | |
| Contact with sick individual | ||||||
| Yes | 25 | 89.3 | 12 | 42.9 | .001 | 11.11 |
| No | 3 | 10.7 | 16 | 57.1 | 2.7–45.61 | |
| Visited healthcare centrea | ||||||
| Yes | 28 | 100 | 26 | 92.9 | .23 | 5.37 |
| No | 0 | 0 | 2 | 7.1 | 0.24–117.24 | |
Applying the parsimony principle, we chose the 2 variables that were significant in the simple logistic regression analysis: a history of contact with a known case (P<.001) and attending a childcare centre (P=.006). We added the interaction of both variables to the final model, which showed that the risk of contracting HFMD in patients attending a childcare centre that also had contact with a known case was 14 times greater (OR=13.8; 95% CI, 3.79–50.18).
Nail loss occurred in 46.6% of the HFMD cases, with a mean time of 56 days elapsed between the onset of symptoms and the development of onychomadesis, with a minimum of 12 and a maximum of 123 days. We looked for factors that may account for the development of this complication (Table 3). We found an association between onychomadesis and mouth ulcers (OR=2.53; 95% CI, 1.19–3.38). We did not find statistically significant differences based on sex (P=.28) or age (P=.77).
Risk factors for the development of onychomadesis analysed in the study.
| Onychomadesis | Yes | No | χ2 | OR | ||
|---|---|---|---|---|---|---|
| N | % | N | % | P | 95% CI | |
| Fever | ||||||
| Yes | 10 | 47.7 | 11 | 52.4 | .82 | 1.21 |
| No | 3 | 42.9 | 4 | 57.1 | 0.21–6.8 | |
| Anorexia | ||||||
| Yes | 10 | 52.6 | 9 | 47.4 | .33 | 2.22 |
| No | 3 | 33.3 | 6 | 66.7 | 0.42–11.6 | |
| Pharyngitis | ||||||
| Yes | 11 | 57.9 | 8 | 42.1 | .07 | 4.81 |
| No | 2 | 22.2 | 7 | 77.8 | 0.78–29.6 | |
| Upper respiratory tract infection | ||||||
| Yes | 3 | 27.3 | 8 | 72.7 | .10 | 0.26 |
| No | 10 | 58.8 | 7 | 41.2 | 0.05–1.35 | |
| Vesicles/ulcers in throat | ||||||
| Yes | 11 | 68.9 | 5 | 31.3 | .006 | 11 |
| No | 2 | 18.7 | 10 | 83.3 | 1.73–69.95 | |
| Vesicles/ulcers on hand | ||||||
| Yes | 9 | 45 | 11 | 55 | .81 | 0.81 |
| No | 4 | 50 | 4 | 50 | 0.15–4.22 | |
| Vesicles/ulcers on foot | ||||||
| Yes | 11 | 57.9 | 8 | 42.1 | .07 | 4.81 |
| No | 2 | 22.2 | 7 | 77.8 | 0.78–29.6 | |
| Vesicles/ulcers in nappy area | ||||||
| Yes | 10 | 50 | 10 | 50 | .54 | 1.66 |
| No | 3 | 37.5 | 5 | 62.5 | 0.31–8.92 | |
The sequencing RT-PCR was positive in only 6 cases. We analysed the sequences of the 268–507 bp-long fragments from these 6 cases. In case 5, the analysis identified the Coxsackie A16 enterovirus. Sequence homology between the enteroviruses of the outbreak, when compared to one another, was 99% to 100%, and there was 97% homology between these and the sequences available for Coxsackievirus in the GenBank.
DiscussionWe consider that the main source of bias in our study was memory bias, as the earliest cases arose in September 2011 and the interviews were conducted in March 2012. The alarm the outbreak generated among families may have worked in our favour, contributing to a more accurate recollection of the signs and symptoms presented by their children. On the other hand, we may have underestimated the number of cases, as patients that did not visit their paediatrician at the Peligros UGC or that sought care in a different Primary Care UGC, an emergency department or a private clinic were not included in the study. Thus, we could not calculate incidence rates to assess whether there was a greater-than-expected number of cases, although we must take into account that providers are not obligated to report cases of HFMD.
This outbreak occurred at the time of year that such outbreaks tend to occur in childcare centres and in the rest of the general population. Paediatricians detected the increase in cases and considered it was above the expected number, having worked in this BHA for several years.
The clinical features described were characteristic of HFMD.1,2 Gastrointestinal symptoms were not documented, although this does not mean they did not occur. The mean duration of the disease was 6 days. The mean age of the affected patients was 20.8 months, which was not significantly different from that of the control group. These data are consisted with the literature.9 As for the age of the affected children, there was a greater clustering of cases (75%) in patients older than 12 months, which seems logical, as ambulation starts around this age and facilitates the transmission of the disease. The maximum age was less than the one described in the literature,18 which accounts for the risk factors we identified, that is, attending childcare.
The final model for contracting HFMD included 2 circumstances that contributed to getting ill, which were contact with known cases and attending a childcare centre. It seems logical to assume that these two circumstances were the source of the public alarm. They meant that most people knew affected children and that these children had acquired the disease at the childcare centre and not at home. Perhaps it would have been helpful to assess the environment in the childcare centres in greater detail, looking at factors like crowding, the staff's level of training, and lack of hygiene that may have contributed to direct and indirect transmission.
Our analysis of onychomadesis showed a high proportion of cases.1,2 The mean time elapsed between the onset of symptoms and nail shedding may have been slightly lower due to the bias derived from recalling the dates retrospectively. Considering that the patients were young children and the resistance nails may have to infection (if the complication arises from a localised infection), the possibility that mouth ulcers are a risk factor makes sense, as children tend to put their fingers in their mouth. At the time we conducted the survey, in the first and second weeks of March, some patients were still in the period during which this complication could develop, so we could have obtained a higher prevalence of onychomadesis if we had interviewed the families up to 18 weeks after the onset of symptoms, although it would have been less likely to develop as these patients had already gone past the median time.
The isolated Coxsackie A16 virus was the most prevalent causative agent along with Enterovirus 71 in several outbreaks of HFMD described in South East Asia in 2010.5 It was also involved in outbreaks in other European countries such as Hungary19 and Germany.7 In 2011, this virus was involved in a small outbreak in a Croatian childcare centre.20 It would have been ideal to obtain samples from every patient to confirm the presence or absence of coinfection, especially in those that developed onychomadesis, as was observed in another study.17 Nevertheless, the fact that coinfection was not found in this sample suggests that this virus was the sole causative agent.
After the last case in a childcare centre was registered in late February 2012 and twice the maximum incubation time elapsed without new cases arising, the end of the outbreak was declared. There were new cases at later dates, but they were considered endemic.
We may conclude by saying that there was an outbreak of HFMD detected by paediatricians and household members with striking symptoms and associated with the presence of onychomadesis that caused public alarm. The aetiological agent of the outbreak was a Coxsackie A16 enterovirus transmitted through contact with known cases of the disease and in childcare centres.
Emphasis should be placed on hygiene and health measures in childcare centres such as cleaning the centre or any objects contaminated with stools or secretions, handwashing, and others once the first cases of HFMD arise. The natural history of HFMD should be investigated, and specifically the transmission mechanisms in closed spaces and onychomadesis; children should be prevented from putting their fingers in their mouths as much as possible, and children suspected to have HFMD should not be allowed to attend childcare from the onset of fever through the end of the disease; overcrowding of childcare centres should be avoided; and caregivers and parents should be properly educated about HFMD in general and its mechanisms of transmission in particular.
In order to prevent and control outbreaks of HFMD, hygienic and health measures should be implemented early on, including more frequent cleaning of surfaces that may get contaminated with stool or secretions, handwashing, preventing children from putting fingers in their mouth to the extent possible, avoiding overcrowding, and keeping children with suspected disease away from the centre during the contagious period. It is very important that families and childcare workers are educated about the characteristics of the disease and its mechanisms of transmission.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Navarro Moreno E, Almagro López D, Jaldo Jiménez R, del Moral Campaña MC, Árbol Fernández G, Pérez Ruiz M, et al. Brote de enfermedad boca-mano-pie y onicomadesis causado por el virus Coxsackie A16, Granada. An Pediatr (Barc). 2015;82:235–241.