Total splenectomy in sickle cell disease is related to a high risk of fulminant sepsis and increased incidence of other events, which have not been reported in patients with partial splenectomy. In this study we examined the patients with sickle cell disease and partial splenectomy and compared the clinical and laboratory results with non-splenectomised patients.
Materials and methodsWe studied 54 patients with sickle cell disease who underwent partial splenectomy in childhood from 1986 until 2011 at the Institute of Hematology and Immunology. They were compared with 54 non-splenectomised patients selected by random sampling with similar characteristics.
ResultsPartial splenectomy was performed at a mean age of 4.1 years, with a higher frequency in homozygous haemoglobin S (70.4%), and the most common cause was recurrent splenic sequestration crisis. The most common postoperative complications were fever of unknown origin (14.8%) and acute chest syndrome (11.1%). After splenectomy there was a significant increase in leukocytes, neutrophils, and platelets, the latter two parameters remained significantly elevated when compared with non-splenectomised patients. There was no difference in the incidence of clinical events, except hepatic sequestration, which was more common in splenectomised patients.
ConclusionPartial splenectomy was a safe procedure in patients with sickle cell disease. There were no differences in the clinical picture in children splenectomised and non-splenectomised except the greater frequency of hepatic sequestration crisis in the first group.
La esplenectomía total en la drepanocitosis se relaciona con riesgo de infecciones sobreagudas y con aumento de la incidencia de otros eventos, lo que no se ha comunicado en pacientes con esplenectomía parcial. En este estudio se caracterizó a los pacientes con drepanocitosis y esplenectomía parcial, y se comparó el comportamiento clínico y de laboratorio con los pacientes no esplenectomizados.
Materials y métodosSe estudió a 54 pacientes con drepanocitosis sometidos a esplenectomía parcial durante la edad pediátrica, desde 1986 hasta el año 2011, en el Instituto de Hematología e Inmunología. Se compararon con 54 pacientes no esplenectomizados seleccionados por muestreo aleatorio con características similares.
ResultadosLa esplenectomía parcial se realizó a una edad media de 4,1 años, con una frecuencia mayor en la anaemia drepanocítica (70,4%) y su causa más común fue la crisis de secuestro esplénico recurrente. Las complicaciones posoperatorias más frecuentes fueron: fiebre de origen desconocido (14,8%) y síndrome torácico agudo (11,1%). Después de la esplenectomía, aumentaron significativamente los leucocitos, neutrófilos y plaquetas; estos 2 últimos parámetros se mantuvieron elevados de manera significativa cuando se compararon con los pacientes no esplenectomizados. No hubo diferencias en la incidencia de los eventos clínicos, excepto el secuestro hepático, que fue más frecuente en los esplenectomizados.
ConclusiónLa esplenectomía parcial constituyó un proceder seguro en los pacientes con drepanocitosis. No hubo diferencias en el cuadro clínico entre los niños esplenectomizados y los no esplenectomizados, salvo la mayor frecuencia de crisis de secuestro hepático en los primeros.
Sickle cell disease is a multisystemic condition, and splenic complications are among its most prominent manifestations.1,2 Spleen enlargement is common in the first decade of life, but vascular occlusive events and recurrent splenic infarctions lead to autosplenectomy. However, splenomegaly persists in some patients to later ages, and other patients require splenectomy for various reasons, such as recurrent splenic sequestration crises (SSCs), hypersplenism, massive splenic infarction, and splenic abscess.2,3
The most common indication for splenectomy in sickle cell disease is SSC.3,4 Total splenectomy is the classical treatment for SSC in children older than 4 years, but the procedure often carries a high risk of fulminant septicaemia, which may be fatal.5 The risk is greater in younger children, in whom the preferred approach is a transfusion regime until at least 2 years of age. Aiming to avoid or reduce the adverse effects of total splenectomy, the Institute of Haematology and Inmunology (IHI) started to perform partial splenectomies in 1986, with excellent results. The remaining spleen remains functional, at least partially, for a period of time that has yet to be determined, and SSCs do not recur, which supports the indication of partial splenectomy in patients with sickle cell disease.2,4–6
For a long time, total splenectomy was only associated to increased risk of overwhelming infections.7 Recent publications have described an increased incidence of vaso-occlusive pain crises (VOPCs), acute chess syndrome (ACS), and stroke following total splenectomy.8,9 Today it is also associated with thrombotic complications, such as deep-venous thrombosis, especially portal venous thrombosis, and with pulmonary hypertension (PHT).10–12 These and other complications have not been reported in patients with partial splenectomy.2,4–6
Due to all the risks currently known to be associated with total splenectomy, there is a growing interest in partial splenectomy.13 The aim of this study was to characterise the clinical and laboratory features of patients with sickle cell disease that had undergone partial splenectomy, and to compare them with those of non-splenectomised patients.
Materials and methodsWe performed an observational, retrospective, comparative study in patients with sickle cell disease who received ongoing care at the haemoglobinopathies clinic of the IHI between September 2010 and December 2011. The source population consisted of patients with sickle cell disease receiving care at the paediatric and adult departments of the IHI. From it we selected a group of patients aged 1–18 years who had undergone a partial splenectomy. We excluded patients who did not want to participate or whose parents or guardians did not give consent, and patients whose records did not include any postoperative clinical data.
The study included 54 splenectomised patients, so we selected a comparative group of non-splenectomised patients with similar characteristics (age, sex, type of haemoglobinopathy) subject to the same exclusion criteria.
We reviewed the medical histories to collect demographic data and postoperative clinical data pertaining to sickle cell disease. We calculated the annual mean counts of haemoglobin, reticulocytes, leukocytes, neutrophils, and platelets, for both before and 6 months after splenectomy. We recorded baseline values for patients (when they had been without transfusions for at least 3 months and without acute clinical events for at least 1 month). In the group of non-splenectomised patients, the data were collected starting at age 4.1 years, which is the mean age at which patients were splenectomised.
We analysed cerebral blood flow (CBF) measured by transcranial Doppler ultrasound, and values of tricuspid regurgitant jet velocity (TRJV) obtained by echocardiography after splenectomy.
We expressed qualitative variables as absolute and relative frequencies. We analysed them using the Chi-squared test, and calculated the odds ratio (OR). Quantitative variables were summarised as mean±standard deviation, and analysed using ANOVA for comparing means and Student's t test for matched pairs. We determined the frequency of events per 100 patients per year. The data were analysed with the statistical software SPSS version 12.0.0. We set the confidence level at 95% and the statistical significance at P≤.05 for all the tests performed.
Ethical considerationsWe adhered to the ethical principles of the Helsinki Declaration. We asked all parents, guardians, and patients for informed consent. The study protocol was reviewed and approved by the ethics committee and the scientific board of our hospital.
ResultsThe study included a total of 54 patients that underwent partial splenectomy, and 54 non-splenectomised patients. Patients with sickle cell anaemia accounted for 70.4% of the splenectomies, HbSβ0 thalassaemia patients for 20.4%, and HbSβ+ thalassaemia patients for 9.3%. Male patients predominated in both groups, accounting for 66.7% of splenectomised patients. The mean age was 15.1 years (range, 1.7–33.7 years) and the duration of follow-up in the clinic was 9.6 years for the group of splenectomised patients. The groups were comparable (Table 1).
Demographic and phenotypic data of the studied population.
| Variables | Splenectomised n=54 | Non-splenectomised n=54 | P |
|---|---|---|---|
| Phenotype SS, n (%) | 38 (70.4) | 43 (79.6) | .266 |
| Male sex, n (%) | 36 (66.7) | 34 (63) | .687 |
| Age (years), mean (range) | 15.1 (1.7–33.7) | 15.3 (4.3–36.9) | .924 |
| Duration of follow-up (years), mean (range) | 9.6 (0.3–25.4) | 9.2 (0.3–32.8) | .756 |
The indication for performing a partial splenectomy was hypersplenism in 7.4% of the operated patients, at a mean age of 7.4 years (range, 2.6–15.4 years), and recurrent splenic sequestration in the remaining patients (92.6%) at a mean age of 3.8 years (range, 0.9–13.8 years). The mean age at the time of splenectomy for all patients was 4.1 years.
Postoperative complications developed in 33.4% of the splenectomised patients. The most frequent complications were fever without source (14.8%) and ACS (11.1%). Sepsis developed in 3.7% of these patients, surgical site infections in 1.9%, and subphrenic abscess in another 1.9%.
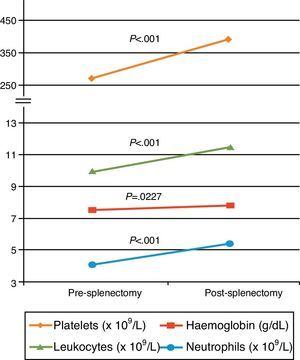
Fig. 1 shows the comparison of haematological variables in patients before and after splenectomy. Following surgery, there was a significant increase (P<.001) in leukocytes (from 9.9 to 11.4×109/L), platelets (from 270.1 to 386.1×109/L), and neutrophils (from 4.1 to 5.4×109/L). Although the increase was not significant, mean haemoglobin rose from 7.55 to 7.86g/dL (P=.227).
The comparison of haematological variables between splenectomised and non-splenectomised patients (Table 2) showed a significant increase in the neutrophil count (5.4 vs 4.8×109/L; P=.026) and platelet count (386.1 vs 342.1×109/L; P=.002) of operated patients. In splenectomised patients, the mean leucocyte count was 11.4×109/L, and in non-splenectomised patients it was 10.6×109/L (P=.060).
Haematological variables in splenectomised and non-splenectomised patients.
| Variable | Splenectomised | Non-splenectomised | P | ||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| Haemoglobin (g/dl) | 7.86 | 7.43–8.3 | 8.0 | 7.73–8.3 | .342 |
| Reticulocytes (%) | 13.9 | 12.7–15.2 | 13.1 | 11.7–14.4 | .319 |
| Leukocytes (×109/L) | 11.4 | 10.8–11.9 | 10.6 | 10.0–11.2 | .060 |
| Neutrophils (×109/L) | 5.4 | 5.1–5.7 | 4.8 | 4.5–5.2 | .026 |
| Platelets (×109/L) | 386.1 | 366.4–405.8 | 342.1 | 323.9–360.3 | .002 |
CI: confidence interval.
Table 3 shows the number of events per 100 patients per year, with similar means found in splenectomised patients compared to non-splenectomised patients: infections (29.0 vs 25.6; P=.559), VOPC (56.7 vs 53.4; P=.821), ACS (14.7 vs 12.9; P=.560), hospital admissions (53.7 vs 40.9; P=.246), and transfusions (39.8 vs 29.1; P=.377). The increase was not statistically significant.
Comparison of clinical events in the splenectomised patient group vs the non-splenectomised patient group.
| Clinical event (×100 patients/year) | Splenectomised | Non-splenectomised | P |
|---|---|---|---|
| Acute chest syndrome | 12.9 | 14.7 | .560 |
| Infection | 29.0 | 25.6 | .559 |
| VOPC | 56.7 | 53.4 | .821 |
| Hospital admission | 53.7 | 4.9 | .246 |
| Transfusion | 39.8 | 29.1 | .377 |
VOPC: vaso-occlusive pain crisis.
Table 4 compares the frequency of other clinical events. Hepatic sequestration crises occurred in 14 (25.9%) of the splenectomised patients, a finding that was statistically significant (P=.041). This clinical event did not appear in either group before age 4.1 years. The differences in the rest of the analysed clinical events were not significant. The mean incidence of SSC was 4.0 before splenectomy, and this clinical event did not appear after surgery. In non-splenectomised patients, the mean incidence of SSC was 0.54 before age 4.1 years, and 0.11 after.
Comparison of other clinical events in the patients under study.
| Clinical event, n (%) | Splenectomised | Non-splenectomised | P |
|---|---|---|---|
| Hepatic sequestration | 14 (25.9) | 5(9.3) | .041 |
| Hepatic crisis | 9 (16.7) | 11 (2.4) | .332 |
| Stroke | 4 (7.4) | 4 (7.4) | 1.0 |
| Priapism | 2 (5.4) | 4 (11.4) | .355 |
| Thrombosis | 2 (3.7) | 1 (1.8) | .558 |
Table 5 shows that the prevalence of a TRJV of 2.5m/s or greater was of 31.8% in splenectomised patients compared to 23.5% in non-splenectomised patients (OR=1.51; P=.56). The mean TRJV was 2.16±0.43m/s in splenectomised patients, and 1.95±0.46m/s in non-splenectomised patients. The CBF velocity measured by transcranial Doppler ultrasound was 170cm/s or greater in 23.5% of splenectomised patients, while these velocities were only seen in 8.8% of non-splenectomised patients (OR=3.18; P=.09). The mean CBF velocity was 137.3±35.4cm/s in splenectomised patients, and 122.6±35.7cm/s in non-splenectomised patients. These results approached statistical significance (P=.09).
Comparison of other clinical variables in the patients under study.
| Variable | Splenectomised | Non-splenectomised | P |
|---|---|---|---|
| TRJV≥2.5m/s,n (%) | Total 227 (31.8) | Total 174 (23.5) | 0.56 |
| TRJV (m/s),mean±SD | 2.16±0.43 | 1.95±0.46 | 0.68 |
| CBF≥170cm/s, n (%) | Total 348 (23.5) | Total 343 (8.8) | 0.09 |
| CBF (cm/s),mean±SD | 137.3±35.4 | 122.6±35.7 | 0.09 |
CBF: cerebral blood flow; SD: standard deviation; TRJV: tricuspid regurgitant jet velocity.
At the IHI, partial splenectomy is a safe and effective procedure for children with sickle cell disease who require it, thanks to advances in immunoprophylaxis, accrued knowledge in the management of the disease, and the experience gained in partial splenectomy since 1986.13
Most splenectomised patients had sickle cell anaemia, the most prevalent type of sickle cell disease in Cuba,4 which is also the most severe form of the disease.1 Following in frequency were patients with HbSβ0 thalassaemia, which is the third most prevalent haemoglobinopathy in Cuba, but has a severe clinical course that cannot be discerned from that of sickle cell anaemia,1,4 so that its manifestations are analysed in combination with those of sickle cell anaemia in most studies.14,15
The mean duration of follow-up after splenectomy was 9.6 years, which was similar to follow-up in the non-splenectomy group, a fact that allowed us to make a broader characterisation of the disease. Duration of follow-up is shorter in international studies.2,5,6,8,9,16 We only found 1 study with comparative groups of non-splenectomised patients and patients who had undergone total splenectomy.17
The average age at which splenectomy is performed for SSC ranges from 2.7 to 7.6 years.3,8,18 In our study, the mean age was 3.8 years, with a slightly broader range of 0.9–13.8 years. At the IHI, partial splenectomy is performed after 2 years of age in patients who have had 2 splenic sequestration events. Before this age, patients are treated with a hypertransfusion regime. Partial splenectomy was performed in 13 (24%) patients before 2 years of age, a figure that corresponded to approximately half the cases reported by Kalpatthi et al. for this age group.8 The indications for the procedure were very severe, life-threatening recurrent SSCs that occurred despite the hypertransfusion regime, and early alloimmunisation in 1 patient. The latter child underwent splenectomy before age 1 year because he became alloimmunised against 3 erythrocyte antibodies (C, E, Fya) in the first month of the hypertransfusion regime. These antigens are more frequent in the Caucasian patients that constitute the majority of donors in Cuba, which is the main reason why patients with sickle cell disease become alloimmunised.19
The 13-year-old patient who underwent partial splenectomy had sickle cell anaemia and was being treated with hydroxyurea. Ninety-four percent of patients with sickle cell anaemia progress to functional autosplenectomy by around 5 years of age,20 but patients treated with hydroxyurea may maintain splenic function for longer.7,21 This could account for the recurrent SSCs in older patients.
The mean age of the children who underwent splenectomy for hypersplenism was 7.4 years. Three of the 4 patients splenectomised for hypersplenism were patients with HbSβ thalassaemia, 2 of them with Sβ0 thalassaemia. Splenectomy in patients with hypersplenism resulted in reduced need for transfusions and removed the intra-abdominal pressure exerted by the enlarged spleen.3
Around 20–30% of patients with sickle cell disease develop postoperative complications.13 One of the most frequent immediate complications is ACS.2,5,6,8,16,22 In our study, 33.4% of patients developed postoperative complications, and had a favourable outcome. The most frequent complications were fever of unknown source and ACS. Laparoscopic total splenectomy is associated to a shorter hospital stay and the same postoperative complications.16,18,23 Recently, there have been reports of laparoscopic partial splenectomy procedures in patients with hereditary spherocytosis, sickle cell disease, and focal splenic tumours.18,24,25
Our study found a significant increase in platelets and leukocytes after splenectomy. These results are consistent with the findings of other authors.2,5,6,8,9 Patients with recurrent SSCs may have a degree of hypersplenism, which would account for the increase in platelet and leucocyte counts following splenectomy.6
Kalpatthi et al.8 did not find significant changes in the haemoglobin and reticulocyte counts from before and after total splenectomy. The results of our study were consistent with this. A study of patients with sickle cell anaemia and recurrent SSC found a significant increase in the neutrophil count after total splenectomy.9 In our study, the results of patients with partial splenectomy were similar. The studies on partial splenectomy that we reviewed did not compare haematological variables of splenectomised an non-splenectomised patients.2,5,6,26
Recent studies describe a higher incidence of VOPC, ACS, and stroke in patients with total splenectomy.8,9,17 Other authors suggest that this difference may be due to increased blood viscosity due to leukocytosis and thrombocytosis.9 Yet others believe it is part of the natural course of the disease.8 However, Wright et al.17 compared a group of patients with total splenectomy with a non-splenectomised control group, and found a significantly higher incidence of VOPC and ACS in splenectomised patients.
In this study, clinical events in splenectomised patients were not significantly different from those in the comparison group, although we found a slightly higher average number of infections, VOPC, hospital admissions, and transfusions in the former group. However, there was a significant increase in hepatic sequestration crisis (HSC) after partial splenectomy compared to non-splenectomised patients. It is likely that SSC and HSC have the same pathophysiological basis.
Patients with sickle cell disease are in a chronic prothrombotic state that is exacerbated during acute vaso-occlusive events.11 Current evidence shows that patients with haemolytic anaemias are at higher risk for thromboembolic complications, especially after total splenectomy. The higher frequency of these complications may be due to the greater number of circulating microparticles and of erythrocytes that expose phosphatidylserine on their surface.10,27
The clinical manifestations of thrombophilia in sickle cell disease include venous thromboembolism, in situ pulmonary thrombosis, and stroke, all of which are more frequent in patients who have undergone total splenectomy.11 However, we did not find significant differences between the 2 groups, either in thrombotic events or in stroke, which suggests that the splenic remnant may protect against these complications.
In the context of our study, partial splenectomy was not associated to the development of leg ulcers and priapism, vascular complications that are frequent in sickle cell disease and correlate to the severity of haemolysis,10 but we must take into account that leg ulcers are rare in children.
Total splenectomy is a risk factor for the development of PHT. In patients with beta thalassaemia, PHT has a high prevalence of up to 70%.10 The prevalence in sickle cell disease is about 30% when echocardiography is used as a diagnostic method, determining the TRJV to estimate the pulmonary artery pressure, but it has not been studied in splenectomised patients. A TRJV of 2.5m/s or greater is associated with a poor prognosis in several studies.10,19 In our study, a TRJV of 2.5m/s or greater was 1.51 times more prevalent in splenectomised patients, although this difference was not significant.
One of the predictors of stroke is cerebral blood flow velocity measured by transcranial Doppler ultrasonography.28 We found no difference in the incidence of stroke between the two groups of patients in our study; however, alterations in cerebral blood flow velocity were 3.18 times more frequent in splenectomised patients than in non-splenectomised patients, a difference that neared statistical significance. These results are consistent with what has been reported in the literature.8 The greater frequency of cerebral flow abnormalities in splenectomised patients could be a risk factor for stroke.
This study allowed us to increase our knowledge of the clinical and haematological changes that occur in patients who undergo partial splenectomy. This evaluation of splenectomised patients 26 years after the introduction of this procedure in the IHI reinforces its efficacy and safety, showing no significant increase in the risk of overwhelming infections. The procedure is also likely to protect from thromboembolic events in the long term, and from other complications described after total splenectomy. A clinical trial must be conducted with a larger number of patients to compare the results of these 2 splenectomy methods and thoroughly investigate the finding of liver sequestration following the procedure. The identification of markers of platelet activation, coagulation, and intravascular haemolysis could be important to elucidate some of the results obtained in our study.
Conflicts of interestThe authors have no conflicts of interest to declare.
We want to thank Dr. Alejandro González Otero, for his invaluable help and advice in the elaboration of this work.
Please cite this article as: Gutiérrez Díaz AI, Svarch E, Arencibia Núñez A, Sabournin Ferrier V, Machín García S, Menendez Veitía A, et al. Esplenectomía parcial en pacientes con drepanocitosis. An Pediatr (Barc). 2015;82:228–234.