Pediatric research is key for improving the diagnosis, treatment and prevention of childhood diseases. In Spain, however, this vital area of research remains underfunded and underrepresented compared to both domestic research in the adult population and pediatric research in other countries.
ObjectiveTo identify the main barriers to pediatric research in Spain and propose strategies to strengthen its development and integration into the public healthcare system.
MethodsThe INVEST-AEP working group conducted an analysis of the current state of pediatric research in Spain. The analysis identified barriers related to funding, training, clinical workloads and bureaucracy, based on which the group proposed several potential improvement strategies.
ResultsThe main barriers include limited funding (5% of national grants), insufficient training in research (both in undergraduate and residency programs), substantial clinical workloads with no protected research time and insufficient collaborative networks. The situation is even more critical in primary care, and, in addition, research efforts do not receive the recognition they deserve for professional career advancement or the competitive application process for public positions. Potential solutions were identified, such as improvements in medical and residency training, consolidation of structured research networks, dedicated sources of funding for pediatric research, protected research time, centralization of ethics committees and recognition of research in merit-based evaluations.
ConclusionsUrgent strategies are needed to strengthen pediatric research in Spain, including increased investment, specific training programs and collaborative networks. A skilled pediatrician should seamlessly integrate clinical practice, teaching and research. Pediatricians, institutions, universities, and the central and autonomous community governments in Spain need to actively promote pediatric research to improve child health and to position Spain as a leader in the field.
La investigación pediátrica es clave para mejorar el diagnóstico, tratamiento y prevención de las enfermedades pediátricas. A pesar de su importancia, sigue infradotada y subrepresentada frente a la investigación en adultos en España y frente a la investigación pediátrica en otros países.
ObjetivoIdentificar las principales barreras de la investigación pediátrica en España y proponer estrategias para fortalecer su desarrollo y su integración en el sistema sanitario.
MétodosEl grupo de trabajo INVEST-AEP realizó un análisis del estado actual de la investigación pediátrica en España. Se identificaron barreras en financiación, formación, carga asistencial y burocracia, y se proponen estrategias de mejora.
ResultadosLas principales barreras incluyen la baja financiación (5% de las ayudas nacionales-ISCIII), la escasa formación en investigación (tanto en pregrado como durante la residencia-MIR), la elevada carga asistencial sin tiempo protegido para investigar y la escasez de redes colaborativas. La situación es aún más crítica en atención primaria. Actualmente la investigación carece del reconocimiento suficiente en las oposiciones y en la carrera profesional. Se identificaron modelos de éxito, como mejoras en la formación de estudiantes de medicina y residentes, consolidación de redes estructuradas, financiación específica, tiempo protegido para la investigación, centralización de los comités de ética, y reconocimiento de la investigación en concursos y oposiciones.
ConclusionesEs urgente implementar estrategias para fortalecer la investigación pediátrica en España, incluyendo mayor inversión, programas formativos y redes colaborativas. El pediatra debe integrar asistencia, docencia e investigación. Pediatras, instituciones, universidades y gobiernos deben impulsar la investigación pediátrica para mejorar la salud infantil y posicionar a España como referente.
Research in pediatrics is key to improving the diagnosis, treatment and prevention of childhood diseases and ensuring delivery of care tailored to their specific needs. Children are not miniature adults; their physical, emotional, and physiological development requires specific studies, but the pediatric population continues to be underrepresented in research compared to adults.
In addition to its impact on children's health, research promotes the professional growth of pediatricians by providing them with tools to approach clinical challenges more effectively. It also promotes innovation, reinforces the training of specialists entering the field and improves the quality of pediatric care. Investment in pediatric research is essential in order to guarantee excellence in care delivery and to improve children’s quality of life. It is also a key element in the efforts to prevent chronic disease, sequelae or health deterioration in adulthood. In a context of growing international investment in child health, it is concerning that pediatric research in Spain continues to be marginalized in domestic research and development (R&D) policy. This disconnect between the burden of disease in the pediatric population and the resources invested in pediatric research poses not only a scientific challenge, but also an ethical imperative.
The aim of this article was to identify the gaps, challenges (Table 1) and opportunities in pediatric research in Spain and to propose specific strategies to strengthen it.
Main structural and cultural barriers to pediatric research in Spain.
| Dimension | Identified problem |
|---|---|
| Limited specific funding | Lack of specific grants or prioritization of pediatric projects in general grant calls |
| Deficient training in research | Scarce training during undergraduate education and residency; research is not considered a core competency |
| Lack of dedicated time | High workloads preclude devoting sufficient time to research. Research activity perceived as “voluntary” or “extra” |
| Lack of professional incentives | Research merits undervalued in competitive funding applications and career advancement. Scarcity of competitive and stable research contracts |
| Excessive bureaucracy | Complex and redundant and administrative procedures, with poor or nonexistent coordination between autonomous communities and levels of care |
| Lack of a cross-cutting research culture | Research is not integrated in the medical curriculum, nor is it valued during MIR training or in real-world pediatric practice. |
MIR, medical intern/resident.
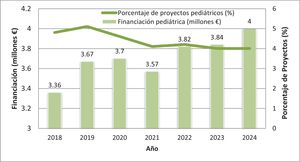
In Spain, clinical research in pediatrics faces significant challenges at both the hospital and primary care (PC) levels, a fact that contrasts with the importance and scope of this specialty within the health care system. Despite its clinical and social relevance, pediatrics is underrepresented in the clinical research field in Spain. Thus, in 2024, only 4% of the projects funded by the Instituto de Salud Carlos III were related to children's health: Of the €100 million awarded in total, roughly €4 million went to pediatric projects. This estimate is based on the review of all the titles and abstracts of the 543 projects funded in 2024 through the Strategic Health Action program of the Instituto de Salud Carlos III (ISCIII), using the key words “pediatría” (pediatrics), “infantil” (pediatric), “niños” (children) and “adolescente” (adolescent).1 Although this methodology has limitations, it provides a relevant estimate for assessing the relative neglect of pediatrics in national funding. These figures have hardly changed over the last five years (Fig. 1). The Strategic Plan of ISCIII for 2021–2025 established infectious diseases, precision medicine and public health as priority areas, but did not specifically mention pediatrics or child health, which reinforces the impression that there is no intended focus on this field.2 At the international level, the Horizon Europe Work Programme, which funds research and innovation projects in different health-related fields explicitly includes the terms “pediatrics” or “children” in less than 3% of the topics open for grant applications since 2021, which also reflects the low priority given to child health in European research policy.3
International comparison: how is Spain doing?In some countries, like the United Kingdom and the United States, pediatric research has been set as a priority, which translates into more funding and greater output. For example, the National Institute for Health and Care Research of the United Kingdom dedicates 10% of its total research budget to pediatric projects, a percentage that is significantly higher compared to Spain.4 In the United States, the National Institute of Child Health and Human Development invests more than $1.4 billion a year in pediatric research.5 These differences are not merely financial, but are also reflected in the structure and organization of research support systems. In the United Kingdom, pediatric clinical trials are integrated into national networks, such as the National Institute for Health Research Clinical Research Network, which devotes around 10% of its budget to pediatric research and also facilitates access to resources and fosters collaboration between centers, including external financial support to supplement the original internal funding of the project. In contrast, investment in Spain remains stable at around 4%–5% of the total health research budget, which positions it below countries with comparable health systems.
Research trainingResearch training in Spain is deficient, as it is not integrated as a cross-cutting competency in the medical curriculum.
The lack of a standardized nationwide curriculum that integrates research training from undergraduate education to specialty training residency programs perpetuates a professional culture that is focused almost exclusively on clinical practice. Research is perceived as an “extra” activity rather than an essential competency for contemporary pediatricians. In the European Union, countries like the Netherlands have integrated research as an absolute requirement for professional advancement, and European curricula for medical specialty training include combined clinical and research training from the residency period. In Spain, without structural reform, we will continue to fail to generate the qualified human resources required to sustain pediatric research in the medium and long term. Integrating research at every level of medical education would yield more qualified physicians and a more innovative health care system.
Clinical research in pediatricsPediatric research faces a number of barriers, among which the excessive workload of physicians is most important, but not the only one. The practice of medicine should integrate care delivery, research and teaching, but the system does always make it possible. It is essential that we foster a health care system committed to excellence, one that balances the different roles of physicians and protects and provides the necessary time and resources to perform them. At present, research is concentrated in teaching hospitals, which have better resources to attract funding and participate in international trials. Institutional structures to conduct research are scarce, and the Río Hortega and Juan Rodés grants, while useful, cannot be the sole pathway to make it possible to combine clinical practice and research. In addition, few of these grants are available, and the health care administrators do not always welcome them, as recipients can only engage in care delivery part-time for the duration of the grant and their activity needs to be subsequently supported by other means. Careers in pediatric research must be promoted by the public health system not only through such grant programs, but by consolidating care delivery schedules to increase efficiency or other approaches. Furthermore, if research were given more weight in the professional advancement of physicians and in the scoring systems applied in hiring processes in the public system, professionals would have incentives to engage in research.
There are many specific challenges in pediatric clinical research, such as obtaining consent from parents or mature minors or assent from children, as appropriate, difficulties in collecting biological samples and the delay in trials of treatments in this age group. Given the low prevalence of many pediatric diseases, collaborations between research groups and networking are essential for obtaining reliable and significant data.
In spite of these challenges, research is a rewarding activity that fulfils those who practice it. However, for it to prosper, it is essential to allocate the necessary time, establish adequate structures and recognize research as a key component in a career in medicine.
Research involving medicines. Pediatric clinical trials. Pediatric research protocolsDifferences in pharmacokinetics and pharmacodynamics between children and adults, which affect the response to treatment and its safety, make pediatric research necessary. Children have unique physiological and developmental characteristics that require specific clinical trials to ensure that health care interventions are age-appropriate.
In addition, many drugs are not originally formulated for pediatric use, so they require adjustments in taste, dose and dosage form. Research needs to be conducted on these modified formulations to assess their bioequivalence, safety and efficacy, as the World Health Organization recognizes.6 In spite of this, only a small percentage of the pharmaceuticals approved by the Food and Drug Administration or the European Medicines Agency have been authorized and undergone trials for pediatric use. A major cultural shift is required the institutional level to change this status quo.
The management of logistic, legal, and cultural aspects poses a major challenge in pediatric clinical trials. Although regulatory agencies, especially in the United States and Europe, have implemented incentives and regulations to promote pediatric research over the past two decades, ethical and operational difficulties remain. Active involvement of minors in drug development is key to ensure that clinical trials take into account their rights, needs and particular characteristics.
Following the introduction of Regulation 1901/2006 of the European Parliament and of the Council on medicinal products for pediatric use,7 there has been an increase in the number of clinical trials with pediatric participants. This regulation demands the development of a pediatric investigation plan for medicinal products developed for adults if the disease or condition for which the product is intended also occurs in children. This provision has facilitated the study and marketing of pediatric drugs, which in turn has improved the safety and efficacy of pharmacological treatments in this age group. However, it is important to continue to promote policies to ensure that pediatric research is prioritized, the allocation the necessary resources and the establishment of a regulatory framework that is efficient in overcoming current barriers and providing adequate therapies to children.
On the other hand, important initiatives have been launched recently, such as the publication of a document of recommendations to increase and improve the participation of pediatric patients in pharmaceutical R&D.8 This guideline was developed by a working group organized by Farmaindustria with the participation of the Red Española de Ensayos Clínicos Pediátricos (RECLIP [Spanish Network of Pediatric Clinical Trials], www.reclip.org), the Asociación Española de Pediatría (AEP, Spanish Association of Pediatrics) and representatives of the Hospital de Sant Joan de Déu de Barcelona (the KIDS Barcelona group and a parents group) whose aim is to ensure participation and consideration of these patients and their families in the drug research and development process to improve their experience and increase their involvement in the investigation of novel treatments. These developments give room for hope in a very specific field of research and target population that wants to, and should, be considered a priority and a routine part of the process.
Research at the primary care levelMore than half of pediatric care providers in Spain work in the primary care (PC) system. Overall (including family physicians), the budget for PC pediatric services does not reach 20% of the total health care budget, but these providers manage 90% of the presenting complaints in the population. It is worth noting that, according to bibliometric studies,9 only 6% of domestic scientific articles in the field of pediatrics listed only PC pediatricians as authors, and a PC pediatrician was the first author in only 13%. The relative impact of these articles (based on the impact factor) is poor, which reflects the current situation in pediatric research. In its 2022–2023 strategic framework the Interterritorial Health Council of Spain highlighted the importance of research in PC, but little progress has been made. This stands in contrast to the substantial potential of the PC system for the performance of epidemiological studies and real-world data, as stated in the ICAP guideline.10
Primary care pediatricians are in a privileged position to conduct research due to their ongoing contact with the pediatric population, healthy and ill alike. Their knowledge of the family environment allows them to study prevalent diseases and incipient chronic diseases and longitudinal studies at the population level. In developed countries, many causes of morbidity and mortality are associated with lifestyle habits. Primary care plays a key role in the study of these factors through the development of health promotion and education strategies. Strengthening research in PC is essential to improve prevention and care quality in pediatrics.
The lack of a national regulatory framework for studies other than clinical trials complicates multicenter projects by requiring authorizations in each autonomous community, which are subject to their corresponding non-standardized regional regulations.11 In addition, excessive clinical workloads, resulting from poor human resource planning, limit the time available for research. Health care administrators do not prioritize research in primary care, as they do not promote specialized units, training or collaborative networks. Countries such as Denmark and the Netherlands have demonstrated in an exemplary manner how the integration of primary care into national research networks significantly improves the quality and quantity of data.12
There is not much of a research culture in PC, and PC professionals themselves do not consider research an essential part of their work. In the MIR training program, there is still a clear imbalance in favor of hospital-based pediatrics, which contributes to future pediatricians undervaluing PC, including research work at that care level. The invisibility of PC in domestic research funding circuits is alarming. This institutional gap reproduces the existing inequalities between levels of care, marginalizing a majority of pediatricians in Spain to the fringes of the knowledge-generating ecosystem. Specific mechanisms for PC are required: adapted grant applications, networks of clinician-researcher mentorship and horizontal structures with “in-house” methodological support. Neglecting to do this is condemning PC pediatrics to a structural form of scientific irrelevance.
Ethics in pediatrics. Ethics committeesGood clinical practice requires knowledge of the regulations applicable to any research involving human subjects, as established in the Nuremberg Code,13 and outlined in the Declaration of Helsinki of the World Medical Association and its successive updates, the latest of which was adopted in Helsinki in October 2024.14 Any study in minors requires the authorization of an ethics committee and, where applicable, informed consent. Since minors cannot legally provide consent, consent must be given by their parents or guardians. Pediatric research involves special considerations to ensure child protection and respect for children's rights. It is crucial for society to understand the importance of research in improving children's health. Pediatricians must convey how these studies contribute to medical advancement. Parents must understand that participating in a clinical trial is not dangerous, but rather an opportunity to contribute to scientific progress.
Pediatrician-scientists require knowledge of medical ethics and should only engage in ethical research. What constitutes appropriate research when children are involved? Foster15 recommends that ethics committees asks the following questions: Does the project ask an important question? Will the project help answer this question? Are the risks to the research subjects acceptable? Will the research subject’s autonomy be respected by obtaining consent? The first two apply to clinical research in general. The last two are particularly relevant to research involving children, and their application varies with age.
A demand that researchers have been advocating for as a collective is the requirement of a single and binding decision for any biomedical study. This strategy was recently adopted by consensus through the Federación de Asociaciones Científico Médicas Españolas (FACME, Federation of Spanish Medical-Scientific Associations),16 with substantial representation of institutions, research networks and scientific societies.
The legislation on clinical trials of pharmaceuticals included in Royal Decree 1090/2015 of December 417 and Royal Decree 957/2020 of November 318 both regulate observational studies involving medicinal products for human use and stipulate that there should be a single evaluation by a research ethics committee and the resulting decision should be binding.
Justifying additional ethics evaluations at the local level on the basis of the Law on Biomedical Research18 does not seem warranted. This very law specifies, among the functions of ethics committees, the responsibility to “coordinate their activity with that os similar committees in other institutions”, and article 16 specifies, in relation to the evaluation by a research ethics committee, that “in the case of research projects carried out in more than one center, the homogeneity in evaluation criteria will be guaranteed, and a single ruling will be produced.” These stipulations are incompatible with requiring that biomedical studies be evaluated repeatedly, giving rise to different decisions and multiple rulings.
It is not reasonable to apply more stringent conditions to studies that do not involve drugs compared to those that do. Provided that it is conducted by an accredited research ethics committee, there should be a single evaluation and the resulting decision should be binding throughout the Spanish territory, thus guaranteeing a standardized process for multicenter biomedical research, whether it involves drugs or not. Spanish legislation allows for a single ruling, but fragmented and inefficient practices remain that are profoundly discouraging for researchers. This fragmentation contravenes the principles of equity, transparency and efficiency that should govern clinical research in the pediatric population.
The role of emerging technologies. Artificial intelligenceArtificial intelligence (AI) increases efficiency and decreases costs in medical research, which is particularly useful in pediatrics.19,20 It can estimate appropriate doses and help select appropriate formulations, model the pharmacokinetics of new therapies without invasive tests and predict differences in efficacy and effectiveness.21,22
In addition, machine learning helps predict diseases and analyze health data, while deep neural networks optimize the interpretation of imaging tests. Natural language processing facilitates the analysis of health records and the automation of reports. Artificial intelligence is also transforming medical training with simulation-based and adaptive learning, thus revolutionizing pediatric care.
However, their implementation poses challenges in relation to privacy, bias and the need for adequate regulation.23 In Spain, regulations such as the 2024 Spanish Strategy for Artificial Intelligence have been established to guarantee the ethical and safe use of AI in the medical field.24,25 With the ongoing development of AI and the integration of large volumes of data, its impact on the prevention, diagnosis and treatment of diseases continues to grow, improving medical care and research. However, its implementation in pediatrics requires specific data, the generation of which is currently inadequate in Spain on account of the relative weakness of pediatric clinical research systems.
Strategies to promote pediatric research. Proposed improvement strategiesDespite the challenges faced by pediatric research, implementing proactive strategies can improve it. The fact that medical societies and journals are raising awareness of the problem is already a significant step in the right direction.
Medical education must be reformed, health care administration improved and institutional and governmental support provided so that pediatric research can have the standing it deserves. Likewise, pediatricians should take an active role in public health research, leading studies that serve as examples for other specialties. With commitment and structural support, pediatric research can be strengthened and have a greater impact.
Table 2 presents an analysis of the main problems and proposes some strategies to address them.
Identified barriers and possible strategies to improve pediatric research.
| Area | Problem | Cause | Strategy | Rationale |
|---|---|---|---|---|
| Training | Lack of research skills | Lack of structured training in undergraduate education and the medical residency | Introducing compulsory and optional research modules in undergraduate and MIR programs. Promoting the undertaking of doctoral theses in MIR training | Incentivizing the interest in research from early on and building research capacity from the ground up |
| Funding | Relative scarcity of funding for pediatric research | Lack of recognition and strategic prioritization of pediatric research by funding agencies | Including pediatrics as a strategic line. Creating specific grants. Increasing allocation of resources for pediatric research. Participation of pediatricians in decision-making bodies | Improving access to resources and promoting relevant projects in child health |
| Workloads | Lack of protected time for research | Among administrators, lack of awareness of the importance of integrating research | Restructuring of care delivery duties to protect dedicated research time. Providing incentives for conducting research. | Recognizing research as part of routine medical practice |
| Integration in primary care | Greater difficulty in conducting research Lack of resources, bureaucratic barriers | Low awareness of the importance of research among pediatricians and administrators | Promoting specific primary care research networks. Specialized administrative, methodological and managerial support. Simplifying bureaucracy. Collaborative primary care-hospital-based work (RECs, etc) | Harnessing the potential of this level of care and its privileged access to the general pediatric population |
| Ethics and Research Committees | Insufficient knowledge among pediatricians. Complex and often repetitive procedures. | Dispersion of committees across regions and between hospitals and primary care, without connection | Advancing toward the establishment of a single REC decision. Simplifying the administrative procedures required for collaboration of different autonomous communities and care levels | Promoting ethical, high-quality research in all care settings |
| Infrastructure | Insufficient infrastructure in primary care centers and hospitals | Limited resources and dispersion of competencies | Creating research units at every level of care with free support for researchers (methodological, statistical, etc). Pediatric clinical trial units. | Facilitating access to research |
| Collaborative networks | Need to join efforts to achieve an adequate number of patients | Lack of structures, formal recognition and funding | Promoting pediatric networks and cooperative studies Specific institutional support for RECLIP and others | Increasing the size of the population available for research. Improving efficiency and impact of collaborative studies |
| Incentives | Research not appealing from a career perspective | Precarious contracts, without recognition in pay scales or economic benefits | Professional and financial incentives. Evaluation of scientific merits in hiring processes. | Encouraging research and retaining talent in pediatric research |
| Visibility | Scarce visibility of pediatric research | Limited access to publication channels. Lack of specific platforms. | Creation of platforms for disseminating pediatric content. Support for open access publications. Promoting activities to disseminate research to the general public. | Increasing the social and professional impact of pediatric research |
REC, research ethics committee; MIR, medical intern/resident; RECLIP, Pediatric Clinical Trial Network.
Pediatric research in Spain is in dire need of support in order to, at least, bring it to the level of other European countries. An educational reform is of the essence, as is raising awareness among administrators and funders and promoting a research culture among professionals. Implementing these changes will improve child health and strengthen the position of Spain as a reference in pediatric research and, by extension, of excellence in pediatric care. This challenge is not merely technical or economic, but also political and strategic: we must decide whether or not the pediatric population is a research priority in our country.
Annex 1: Members of the INVEST-AEP platformTalía Sainz, Department of Pediatrics and Infectious and Tropical Diseases, Hospital Universitario La Paz; Fundación IdiPaz, Universidad Autónoma de Madrid; Biomedical Research Networking Center for Infectious Diseases (CIBERINFEC), Instituto de Salud Carlos III, Madrid, Spain.
Begoña Santiago, Section on Infectious Diseases, Hospital Universitario Gregorio Marañón, CIBERINFEC, Madrid, Spain.
Antoni Soriano-Arandes, Serveis de Salut Integrats del Baix Empordà, Palamós, GErona, Institut de Recerca Vall d’Hebron, VHIR, Barcelona, Spain.
Quique Bassat, ISGlobal, Hospital Clínic, Universitat de Barcelona, Barcelona, Spain.
Gemma Olivé-Cirera, Hospital de la Santa Creus i Sant Pau de Barcelona, Institut de Recerca de Sant Pau, Barcelona, Spain.
Daniel Natera-de Benito, Neuromuscular Unit, Department of Neurology, Hospital Sant Joan de Déu, Barcelona, Applied Research Group on Neuromuscular Diseases, Institut de Recerca Sant Joan de Déu, Barcelona, Biomedical Research Networking Center for Rare Diseases (CIBERER), Instituto de Salud Carlos III, Madrid, Spain.
Juan Manuel Rius-Peris, Hospital Universitario Virgen de la Luz, Cuenca, Research Group in Electronic, Biomedical and Telecommunication Engineering, University of Castilla-La Mancha, Cuenca, Research Group on Biomedical Engineering, Instituto de Investigación Sanitaria de Castilla-La Mancha (IDISCAM), Spain.
Miguel Tortajada-Girbés, Section of Pediatric Allergy and Pulmonology, Hospital Universitario y Politécnico La Fe, Valencia; “Childhood Allergy and Respiratory Diseases” accredited research group. Instituto de Investigación Sanitaria La Fe de Valencia. Department of Pediatrics, Obstetrics and Gynecology, School of Medicine, Universitat de València, Spain.
Asunción Mejías, Department of Infectious Diseases, St. Jude Children’s Research Hospital, Tennessee, United States.