Acute focal bacterial nephritis is an interstitial bacterial infection, localised in the renal parenchyma, which can be more serious than acute pyelonephritis. The aim of this study is the analysis of predictive factors that may lead to its early diagnosis, which is essential for an adequate therapeutic approach.
Patients and methodsA retrospective, multicentre case and control study. The participant centres were hospitals in Castellon and Valencia. The study period was 2010–2018, with the cases being patients with focal bacterial nephritis and the patients with pyelonephritis as controls.
ResultsA total of 158 (1:1) patients were included. The median age of the cases was 2 years and there were 75% females. There were no differences in the clinical presentation. In the univariate analysis, focal nephritis was associated with malformations of the urinary tract, bacteraemia, the neutrophil count, and procalcitonin, as well as febrile convulsions of borderline significance. Procalcitonin values ≥2 ng/mL had an OR of 4.9 (95% CI; 1.77–13.85) of presenting with focal nephritis. In the multivariate analysis, the urological malformations still maintained statistical significance and borderline significance for procalcitonin.
ConclusionsThe urinary tract malformations predispose the development of focal bacterial nephritis. In patients with a urinary tract infection and predictive factors of acute focal bacterial nephritis it would be worthwhile performing a renal Doppler ultrasound in the acute phase for its appropriate diagnosis and treatment.
La nefritis focal bacteriana aguda es una infección intersticial bacteriana, localizada en el parénquima renal, que entraña mayor gravedad que la pielonefritis aguda. El objetivo del estudio es el análisis de factores predictivos que permitan su diagnóstico precoz, fundamental para un adecuado abordaje terapéutico.
Pacientes y métodosEstudio multicéntrico de casos y control retrospectivo. Centros participantes: hospitales de Castellón y Valencia. Periodo de estudio: 2010-2018. Casos: nefritis focal bacteriana. Controles: pielonefritis aguda.
ResultadosSe incluyó a un total de 158 pacientes (1:1). La mediana de edad de los casos fue 2 años. El 75% de sexo femenino. No existieron diferencias en la presentación clínica. En el análisis univariante la nefritis focal se relacionó con malformaciones del tracto urinario, bacteriemia, recuento de neutrófilos y la procalcitonina, así como las convulsiones febriles en el límite de la significación. Valores de procalcitonina ≥ 2 ng/mL tiene una OR de 4,9 (IC del 95: 1,77-13,85) de presentar nefritis focal. En el análisis multivariante las malformaciones urológicas mantuvieron la significación estadística y la procalcitonina en el límite de la significación.
ConclusionesLas malformaciones del tracto urinario predisponen al desarrollo de nefritis focal bacteriana. Ante pacientes con infección del tracto urinario y factores predictivos de nefritis focal bacteriana aguda, sería recomendable la realización de una ecografía Doppler renal en fase aguda para un diagnóstico y un tratamiento adecuado.
Urinary tract infections are among the most frequent bacterial diseases in the paediatric age group and usually have favourable outcomes with early antibiotherapy. However, there are forms that may have a more severe course, such as acute focal bacterial nephritis (AFBN), renal abscess and pyonephrosis.
Acute focal bacterial nephritis, also known as acute lobar nephronia (ALN), is an interstitial bacterial infection of the renal parenchyma that may involve one or more renal lobes. Initially, it was believed that its incidence is low in children, but advances in the resolution of imaging tests and increased awareness of the disease have led to an increase in its diagnosis, and it is now believed that the disease continues to be underdiagnosed. It was described in 1979 by Rosenfield et al.1 in adults and children, although the first case reported in the paediatric literature was published in 1985 by Lawson et al.2 The histological examination reveals hyperaemia, interstitial oedema with leukocytic infiltration and without necrosis and liquefaction.3 Patients with AFBN usually present with high fever and rapid deterioration of general health status and it is clinically indistinguishable from acute pyelonephritis (APN), although in some cases it may mimic an abdominal inflammatory process, such as acute appendicitis or even a tumour, which may result in delayed diagnosis and treatment. On ultrasound, it appears as a localised lesion in the renal parenchyma with poorly defined borders that is hypoechoic, with reduced perfusion and poor corticomedullary differentiation, possibly associated with kidney enlargement, which requires ruling out a renal abscess or tumour. However, depending on the stage of AFBN, lesions go from being hyperechoic to being isoechoic or hypoechoic.3,4 Although renal ultrasound offers a lower sensitivity and specificity compared to computed tomography (CT),3,5 some authors have reported a sensitivity of renal Doppler ultrasound of 89% in their case series.5,6 On CT, AFBN appears as poorly demarcated areas with a wedge shape that are not enhanced after infusion of contrast.7 Computed tomography is indicated in case of uncertain diagnosis or a poor response to appropriate antibiotherapy.8,9
The pathogenesis of AFBN is still under debate, with proposed routes of infection including ascent from the lower urinary tract and haematogenous spread. Hitherto considered an intermediate stage in the spectrum ranging from APN to renal abscess,10 some authors are now discussing whether it is not a separate entity.11 Early detection is important in order to initiate intravenous (IV) antibiotherapy, with a longer-than-usual duration in order to minimise the risk of renal scarring, which is greater in this disease.12
The aim of our study was to analyse clinical and testing factors that could predict AFBN in paediatrics and thus allow early diagnosis and appropriate treatment to minimise the risk of renal scarring.
Material and methodsStudy design: retrospective multicentre case-control study.
Period under study: January 1, 2010–December 31, 2018.
Sample: inclusion criteria—case: patients aged 1 month to 14 years with a diagnosis of AFBN admitted to the Department of Paediatrics of the Hospital General Universitario in Castellon, Hospital Universitario La Plana, Hospital Comarcal in Vinaroz, Hospital Clínico Universitario in Valencia and Hospital Universitario Politécnico La Fe in Valencia. Control: patients aged 1 month to 14 years with a diagnosis of APN. In adherence to the principles of comparability that apply to case-control studies, we ensured that the age distribution and dates of admission of controls were similar to those of cases by matching patients in a 1:1 design.
Exclusion criteria: transfer to a different hospital; lack of subsequent follow-up by the departments of paediatrics or paediatric nephrology; congenital or acquired immunodeficiency; transplant recipient; presence of other diseases that the researchers deemed a potential source of bias in the analysis.
Sample size: we calculated that the number of cases and controls needed to detect an odds ratio (OR) of 3 in clinical factors with an assumed frequency of exposure in controls of 30%, a risk of alpha error of 5% and a power of 80% was 64 cases and 64 controls.
Methods: retrospective review of electronic and paper-based health records of paediatric patients with a diagnosis of AFBN and APN. We defined APN as fever (>38.5 °C) of abrupt onset with or without associated symptoms such as urinary symptoms, lumbar or abdominal pain with tenderness elicited on fist percussion over the kidney, irritability or vomiting, and laboratory findings suggestive of bacterial infection such as leukocytosis, elevation of C-reactive protein (CPR) or procalcitonin (PCT) in isolation or combined. Urine test positive for leukocyte esterase or nitrite or with a white blood cell (WBC) count greater than 10 cells/high-power field (HPF, 400×). The diagnosis of APN required microbiological confirmation with a significant bacterial count depending on the method of sample collection or evidence of renal parenchymal involvement in imaging tests. We did not require confirmation by dimercaptosuccinic acid (DMSA) renal scintigraphy.
We defined acute focal bacterial nephritis based on sonographic findings (focal hyper- or hypoechoic lesion with poor perfusion, irregular and poorly demarcated margins, possibly associated to significant renal enlargement) or CT findings (poorly demarcated areas with a wedge shape that are not enhanced after infusion of contrast). Renal ultrasound and CT scans were interpreted in the departments of radiology of participating hospitals. We did not require confirmation by CT.
We analysed epidemiological, clinical, laboratory and treatment variables.
Statistical analysis: descriptive analysis with expression of quantitative variables as mean and standard deviation in case of a normal distribution and median and interquartile range otherwise, and expression of qualitative variables as proportions. For the inferential analysis, we performed a univariate analysis comparing quantitative data by means of the Student t test or Mann–Whitney U test for independent samples as applicable, and categorical data by means of the χ2 test or Fisher exact test. We used the Shapiro–Wilk test to assess the normality of distributions. We conducted a multivariate analysis by means of logistic regression. To calculate the OR for categorical variables in case of blank cells, we applied a proportional continuity correction. We assessed diagnostic accuracy by means of receiver operating characteristic (ROC) curve analysis. We defined statistical significance as a two-sided p-value of less than 0.05 for all tests. The statistical analysis was performed with the software package Stata 13.0.
Ethical considerations: the study protocol was reviewed and approved by the Research Ethics Committee of the Hospital General Universitario de Castellón. Both the principal investigator and all other researchers declared no conflicts of interest. The study was conducted without funding from external sources.
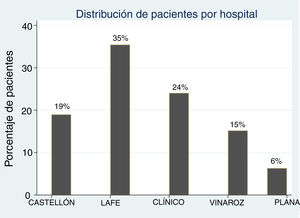
ResultsDescriptive analysis: the study included a total of 158 patients (79 cases and 79 controls). Fig. 1 presents the distribution of patients by hospital. The median age of cases was 2 years (IQR, 0.84−4.85; range, 0.11–13.29); 75% were female. Of all cases, 33% (n = 26) had congenital anomalies of the kidney and urinary tract (CAKUT), of which vesicoureteral reflux (VUR) was the most frequent malformation, found in 65% (n = 17), 35% (n = 8) had duplex kidney/bifid renal pelvis, 15% (n = 4) hydronephrosis, 1 patient had kidney hypoplasia and 1 ureterocoele. Not all cases and controls underwent a voiding cystourethrogram (VCUG).
The mean maximum temperature in the cases was 39.5 °C (standard deviation [SD], 0.57 °C). As for the laboratory results, the mean WBC count was 20 581 cells/mm3 (SD, 6984), the median neutrophil count 14 050 cells/mm3 (IQR, 9940–17 930), the median level of CPR 134.2 mg/L (IQR, 74–243.8) and the median level of PCT 4.27 ng/mL (IQR, 0.93–19.7). Table 1 summarises the rest of the clinical and laboratory features.
Univariate analysis of predictors of acute focal bacterial nephritis.
| Variables | Cases | n | Controls | n | P |
|---|---|---|---|---|---|
| Epidemiological variables | |||||
| Age (years) | 1.98(IQR, 0.84−4.85) | 79 | 1.90(IQR, 0.84−3.81) | 79 | .888a |
| Female sex | 74.6% | 79 | 74.5% | 79 | .573b |
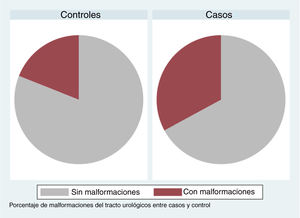
| Malformations | 32.9% | 79 | 17.7% | 79 | .028b |
| Clinical variables | |||||
| Fever (maximum temperature) | 39.53 (SD, 0.57) | 79 | 39.44 (SD, 0.63) | 77 | .366a |
| Days of fever | 4 (IQR, 2−5) | 74 | 3 (IQR, 2−5) | 77 | .684c |
| Vomiting | 38% | 79 | 41.8% | 79 | .626b |
| Pain on side | 17.7% | 39 | 21.5% | 39 | .642b |
| Urinary complaints | 10.1% | 39 | 20.2% | 39 | .082b |
| Febrile seizures | 6.3% | 79 | 0% | 79 | .058d |
| Urine sediment | |||||
| Leukocyturia | 91.1% | 79 | 97.4% | 79 | .086b |
| Nitrites | 43% | 79 | 39.2% | 79 | .628b |
| Haematuria | 77.2% | 79 | 78.5% | 79 | .848b |
| Proteinuria | 69.6% | 79 | 64.5% | 79 | .498b |
| Bacteriuria | 63.3 | 79 | 76% | 79 | .084b |
| Blood tests | |||||
| Blood culture | 8.8% | 79 | 0% | 79 | .013d |
| White blood cell count (/mm3) | 20 581 (SD, 6984) | 77 | 18 995 (SD, 7.320) | 78 | .169a |
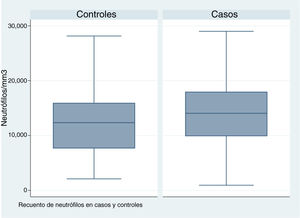
| neutrophil count (/mm3) | 14 405 (SD, 6070) | 77 | 12 422 (SD, 6.056) | 78 | .043a |
| CPR (mg/L) | 152.1 (IQR, 74−243.8) | 78 | 116.6 (IQR, 54−193.6) | 76 | .329c |
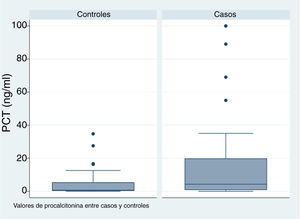
| PCT (ng/mL) | 4.2 (IQR, 0.93−19.7) | 47 | 0.7 (IQR, 0.4−5.28) | 37 | .009c |
When it came to the aetiological agent, Escherichia coli was the species isolated most frequently, identified in 83% of cases, followed by Enterococcus faecalis and Klebsiella pneumoniae in 3%, Proteus mirabilis in 1.3% and other aetiological agents in 11%. Only one patient had a negative urine culture, with a blood culture positive for Pseudomonas aeruginosa and confirmation of diagnosis by DMSA renal scan. In 78.2% of the cases, patients received an antibiotic agent in monotherapy during the hospital stay, most frequently IV cephalosporins (48.1%), IV aminoglycosides (38%) and IV amoxicillin-clavulanic acid (27.9%). The median duration of antibiotherapy was 14 days (IQR, 13–19). The median length of stay was 6 days (IQR, 4–7) and the median duration of fever was 4 days (IQR, 2−5).
Analysis of potential predictors of AFBN: Table 1 presents the results of the univariate analysis comparing cases and controls. The univariate analysis found statistically significant differences in association with the presence of renal anomalies and malformations of the urinary tract, bacteraemia, the neutrophil count and the level of PCT, while the p-value for the presence of febrile seizures neared the statistical significance threshold.
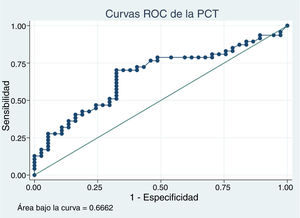
In patients with urinary tract malformations, the OR for having AFBN was 2 (95% confidence interval [CI], 1–4.699) (Fig. 2), compared to an OR of 11.78 in patients with febrile seizures (95% CI, 0.638–215.963) and an OR of 16.45 in patients with bacteraemia (95% CI, 0.922–293.116). When it came to laboratory variables, we found that patients with AFBN had a higher neutrophil count and greater elevation of PCT compared to patients with APN (neutrophil count, 14 050 vs 12 345; PCT, 4.2 vs. 0.7) (Figs. 3 and 4). Based on the ROC curve for PCT, with an area under the curve (AUC) of 0.67, PCT values of 2 ng/mL or greater have a sensitivity of 70.21% and a specificity of 67.6% for diagnosis of focal nephritis, while values greater than 6.4 ng/mL have a sensitivity of 48% and a specificity of 81% (Fig. 5). Values of PCT of 2 ng/mL and higher correspond to an OR of 4.9 (95% CI, 1.77–13.85; P = .001) for the risk of having AFBN.
In the multivariate logistic regression analysis, the only predictor that continued to be significant was the presence of urinary tract malformations, with the p-value for the level of PCT nearing the significance threshold (Table 2).
Multivariate analysis of predictors of acute focal bacterial nephritis.
| Variables | n | P | OR | CI |
|---|---|---|---|---|
| Urinary tract malformations | 82 | .047 | 3.24 | 1.01−10.30 |
| Neutrophil count | .577 | 1 | 0.999−1 | |
| PCT | .064 | 1.04 | 0.997−1.088 |
CI, confidence interval; OR, odds ratio; PCT, procalcitonin. Method: logistic regression analysis.
Acute focal bacterial nephritis, or ALN, is an interstitial infection of the renal parenchymal that is currently underdiagnosed in the paediatric population. In our study, we did not obtain incidence values, but data on the prevalence of AFBN within the total of urinary tract infections have been reported in several studies, with values ranging from 4% to 19% in different studies.8,9,11,13–16 The observed variability in prevalence is mainly associated with the imaging method used for diagnosis. This disease is increasingly identified in children due to the improving resolution of imaging tests and a growing awareness of clinicians.
The multicentre study that we present here is among the studies with the greatest number of cases of AFBN published in the literature. The age distribution was similar in both groups, as this was one of the criteria applied for matching cases and controls, as was the date of hospital admission. The median age in the case group was 2 years, with a range of 0.11–13.29 years. However, based on other studies,15,17 patients with AFBN are older compared to patients with APN. The greater proportion of the female sex in both groups (75%) was consistent with the higher frequency of urinary tract infection in girls and women from age 1 year.
Urinary tract malformations, especially VUR, were more frequent in patients with AFBN (32.9%), and the OR for the risk of AFBN in these patients was double that of patients without malformations. In other case series,9,12,13 the reported prevalence of malformations has ranged from 19% to 72%, although a study published by Chen et al.18 did not identify differences in the proportion of VUR between patients with AFBN and patients with APN. One significant limitation of our study and most other studies in the literature is that a VCUG was not performed in all included patients, so that it was not possible to rule out VUR in all patients, however, due to the invasive nature of this technique and the exposure to ionising radiation that it involves, its use in controls could not be justified from an ethical standpoint.
In cases described in the literature, patients with AFBN present with high fever of abrupt onset and a rapid deterioration of general health.1–4,8,10,13–15,17–20 The disease usually lasts longer compared to patients with pyelonephritis. In our case series, patients had a peak temperature that was similar to that of controls and we did not find any significant differences in clinical features at admission save for the presence of convulsive seizures, which was more frequent in the cases, as described in the previous literature.21,22 However, we found a difference, albeit not statistically significant, in the duration of fever, with more days of fever in the case group. A study by Yang et al. comparing patients with AFBN and patients with APN found that children with AFBN were older, had a longer duration of fever prior to admission, higher body temperatures, a higher WBC count and higher levels of CPR compared to children with APN,17 while a study by Chen et al. with a similar design found that patients with AFBN had higher levels of CPR, were more likely to have nausea or vomiting and had a higher duration of fever after initiation of antibiotherapy.18
In our sample, bacteraemia was more frequent in patients with AFBN, which may be explained by the greater severity of the infectious disease. Kline et al.23 reported a proportion of bacteraemia of 33% in a case series, while other studies have not found an association between bacteraemia and AFBN.4,8,15
When it comes to the laboratory findings reported in the literature, at the time of diagnosis patients with AFBN have a higher WBC count, a higher neutrophil count, a lower lymphocyte and platelet count, higher serum levels of PCT and a higher urine β2-microglobulin to creatinine compared to patients with APN22; in our study, patients with AFBN exhibited higher neutrophil counts and levels of PCT. Procalcitonin values of 0.5 ng/mL ang higher have been associated with the presence of APN compared to acute cystitis, based to a review with metaanalysis.24 In our study, patients with AFBN had higher PCT levels (4.2 ng/mL [IQR, 0.93−19.7] vs 0.7 ng/mL [IQR, 0.4−5.28]). Based on the ROC curve for PCT, values of 2 ng/mL and greater offer a sensitivity of 70.21% and a specificity of 67.6% for diagnosis of focal nephritis, neither reaching the 80% required to consider it a valid diagnostic test. A larger sample would be required to obtain a better fit in the analysis, as we did not have this value on record for every patient included in the analysis.
In agreement with the previous literature, we did not find differences based on the aetiological agent, and E. coli was the species isolated most frequently in both groups, which was consistent with previous case series.3,4,8,9,13,14,17,25 There have been studies in the past in which urine cultures were negative in up to 20% to 25% of cases,12,14 but in our case series, only 1 patient had a negative urine culture.
As concerns the management of patients with AFBN, we ought to highlight that 78.2% of our patients received an antibiotic agent in monotherapy despite the recommendations for biotherapy. The median length of stay was 6 days, while the mean duration of antibiotherapy was 14 days with a range of 7–36 days, as recommended for treatment of AFBN,3 although a study published by Cheng et al. in 2006 found that there was a higher probability of recurrence with 2 weeks of antibiotherapy compared to 3 weeks, especially in patients with complicated AFBN.4
Chief among the limitations of our study are its retrospective design and the use of renal ultrasound as the sole imaging test used for diagnosis of AFBN, given that its results depend on the observer and that it offers a lower sensitivity and specificity compared to CT or magnetic resonance imaging. For this reason, we cannot exclude the possibility of selection bias in the control group, as it could include some cases. However, the exposure to ionising radiation and the sedation required for CT make its use unjustified from an ethical standpoint, especially in patients that are improving. Some authors recommend its use in patients with nephromegaly, renal mass or persistence of fever after 5 days of antibiotherapy.7,11
Acute focal bacterial nephritis carries a higher risk of renal scarring,8,10,12 with an incidence of up to 89% in previous studies,12 especially in patients with elevation of inflammatory markers and greater duration of fever before and after initiation of antibiotherapy.
Clinical prediction rules or laboratory markers of AFBN are needed for the early diagnosis of this disease, especially now that international guidelines for the management of urinary tract infections26–29 recommend oral antibiotherapy and restrict the indications for imaging tests.
In the univariate analysis, we found an association of urinary tract malformations, bacteraemia, neutrophilia and PCT with AFBN. However, the only factor that remained significant in the multivariate analysis was urinary tract malformations. The p-value for PCT neared the threshold of statistical significance. In patients with urinary tract infection with risk factors for AFBN, performance of a renal Doppler ultrasound scan is recommended during the acute phase of disease for the purposes of diagnosis and correct treatment.
Conflicts of interestThe authors have no conflicts of interest to declare.
Jesús Lucas García, Eva García Torres, Vicente Olaya Alamar and Andrea Nos Colom, Paediatric Nephrology, Hospital General Universitario Castellón, Castellón; Manuel Oltra Benavent, Cristina Lozano Zafra and Francesc Caballero Chabrera, Paediatrics, Hospital Universitario Politécnico La Fe, Valencia; Susana Ferrando Monleón, Juan Marín Sierra and Beatriz Guzman Morais, Paediatric Nephrology, Hospital Universitario Clínico, Valencia; María Dolores Rabasco Álvarez, Paediatric Nephrology, Hospital Comarcal de Vinaroz, Castellón; Pilar Benito Julve, Paediatric Nephrology, Hospital Universitario La Plana, Villarreal, Castellón.
Appendix A lists the members of the Working Group for the investigation of acute focal bacterial nephritis (AFBN).
Please cite this article as: Lucas García J, Oltra Benavent M, Ferrando Monleón S, Marín Sierra J, Rabasco Álvarez MD, Benito Julve P. Marcadores predictivos de nefritis focal bacteriana aguda. Estudio multicéntrico casos-control. An Pediatr (Barc). 2020;93:77–83.