Holder pasteurization is the technique used most frequently in milk banks to minimize the risk of transmission of infectious agents. Different pasteurization devices have been described that generally use hot water or air as heat sources. In our study, we analysed the quality of pasteurization achieved with a new automated water-free pasteurizer in a neonatal personalized nutrition unit in which donated milk from mothers of infants delivered at different gestational ages and of different postnatal ages is pasteurized.
Material and methodsWe analysed the temperatures of different phases of pasteurization with 8 external probes distributed evenly throughout the pasteurizer. We applied the optimal range criteria established by the European Milk Bank Association (EMBA) to assess the quality of pasteurization. We also analysed the macronutrient composition of 8 samples of donor human milk of different volumes before and after automated pasteurization.
ResultsWe did not find significant differences in the following parameters under study: time from 58 °C to 62.5 °C, duration of plateau, highest temperature during plateau and length of exposure to temperatures over 58 °C. The macronutrient analysis showed significant changes in fat content but not in protein or lactose content.
ConclusionsHolder pasteurization of human milk with a water-free pasteurizer met the quality standards recommended by the European Milk Bank Association independently of the quantity of milk pasteurized in each bottle and with significant changes in the fat content but not in the protein or lactose content.
La pasteurización Holder es la técnica utilizada más comúnmente en bancos de leche materna para minimizar el riesgo de transmisión de agentes infecciosos. Se han descrito distintos sistemas de pasteurización, que generalmente utilizan agua o aire caliente como fuentes de calor. En el presente estudio se analizó la calidad de la pasteurización realizada con un nuevo pasteurizador automatizado en seco, en una unidad de nutrición personalizada neonatal que procesa leche materna donada de madres de lactantes nacidos a distintas edades gestacionales y de distintas edades postnatales.
Material y métodoSe analizó la temperatura durante las distintas fases de la pasteurización con 8 sondas externas distribuidas uniformemente por todo el pasteurizador. Se aplicaron los criterios óptimos recomendados por la Asociación Europea de Bancos de Leche (EMBA) para monitorizar la calidad de la pasteurización. También se realizó un análisis de macronutrientes en 8 frascos con distintos volúmenes de leche materna donada antes y después de su pasteurización programada.
ResultadosNo se objetivaron diferencias significativas en los siguientes parámetros analizados: tiempo de calentamiento de 58 °C a 62,5 °C, duración de la meseta, temperatura máxima durante la meseta y tiempo de exposición a temperaturas superiores a 58 °C. El análisis de macronutrientes reveló diferencias significativas en el contenido graso, pero no en el proteico o el de lactosa.
ConclusionesLa pasteurización de leche materna mediante un pasteurizador sin agua cumplió los estándares de calidad recomendados por la Asociación Europea de Bancos de Leche (EMBA), independientemente de la cantidad de leche procesada en cada frasco y con cambios significativos en el contenido graso, pero no en contenido proteico o de lactosa.
Human milk banks collect, screen, store, process, and distribute donor human milk (DHM) under optimum conditions following standardised protocols and bacteriological controls to provide DHM to infants whose mothers do not have enough milk of their own for a variety of reasons, especially high-risk preterm infants.1 As the nutrient and amino acid contents of DHM vary depending on the gestational age at birth and postnatal age, personalised allocation of DHM taking into account both gestational and postnatal ages could be a better strategy for feeding preterm infants with different requirements.2
Holder pasteurization of DHM is the gold standard used by milk banks worldwide, as it offers a good balance of microbiological safety and preservation of the nutritional/biological quality of the milk. The process requires rapid heating of the milk to 62.5 °C followed by a 30-min plateau phase s at 62.5 °C and ending with a rapid cooling phase to 4 °C. For the process to be considered effective and safe, temperatures changes must be homogeneous independently of the distribution of bottles in the pasteurizer and the volume of milk in the bottles. Air and water pasteurizers are the most frequently used devices. Recently, new and faster pasteurization and non-pasteurization techniques have been developed and used with good outcomes. Some of the promising results are a decrease in nutrient loss, greater preservation of bioactive components and optimal microbiological safety: ohmic heating, high-pressure processing,3 thermo-ultrasonic treatment4 and high-temperature short-time pasteurization at 72 °C for 5–15 s. Most of these methods are still been investigated or have only been implemented by a few milk banks.5
We describe a new automated device for water-free pasteurization of DHM. We assessed the pattern of pasteurization temperatures using a method similar to one described previously in the literature.6
Material and methodsWe performed a longitudinal observational study to assess pasteurization cycle temperatures in the newly developed pasteurizer. The Beldico PA 45 (International Medical Products, Brussels, Belgium) is a water-free pasteurizer that performs the complete pasteurization cycle with a dry technique (conduction process by contact versus convection with warm air) heating the milk to 62.5 °C, maintaining the set temperature for 30 min and then carrying out an automated rapid cooling phase (<10 °C). The complete pasteurization cycle takes approximately 2 h.
The study was performed at the Personalized Nutrition Unit (PNU) of the Department of Neonatology of the Hospital Gregorio Marañón (Madrid, Spain) in September 2019.
The PNU opened in March 2018 to provide pasteurized DHM exclusively to at-risk infants admitted to the neonatal ward. Human milk was donated exclusively by mothers of infants admitted to Hospital Gregorio Marañón. The PNU collects, screens, stores, processes and distributes donated human milk classified according to the gestational age at birth and postnatal age of the child of the donor at the time of collection with the aim of providing the most suitable pasteurized donor human milk (PDHM) for the gestational and postnatal ages of the recipients. Donor milk is not pooled and traceability is maintained throughout the process. Thus, the PNU can provide 9 different types of PDHM: colostrum, transitional and mature milk, each in 3 gestational age ranges (<28 weeks, 28–32 weeks and 32–37 weeks).
As a secondary objective we analyzed the macronutrient composition of different amounts of human milk before and after pasteurization.
SampleThe Beldico PA 45 pasteurizer has 3 independent heating blocks with a “reference bottle” control in each. It is equipped for multichannel processing with a program controller that is based on continuous readings from seven probes. In each of the blocks, a reference bottle serves to continuously monitor the temperature of the milk and drive the pasteurization process, while another 3 probes continuously monitor the temperature of the heating blocks and one last probe continuously monitors the temperature of the cooling fluid.
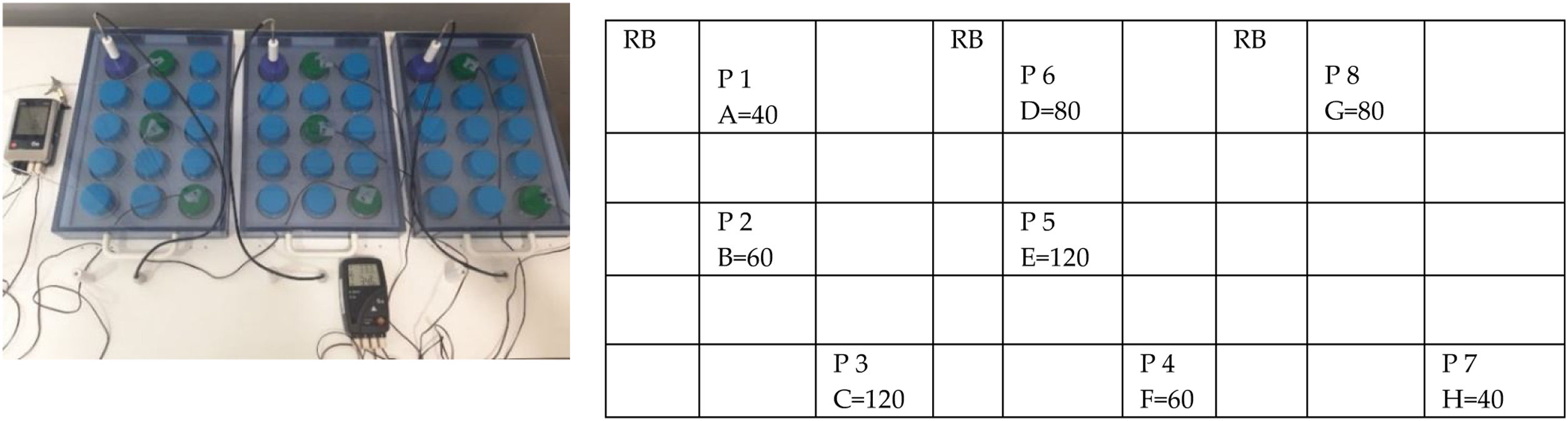
In our study, we continuously monitored temperatures with 8 external probes (Testo® electronic measurement technology) uniformly distributed across the 45 bottles of the pasteurizer (Fig. 1). We run the cycle 3 times after adding different volumes to the bottles: 120 mL of cow’s milk in the first cycle, 60 mL in the second cycle and a volume ranging from 40 to 120 mL in the third cycle (mixed volumes).
Before and after the automated pasteurization of thawed mature human milk from a single donor, we analysed 8 samples from 8 bottles with different volumes between 40 and 120 mL using Fourier transform infrared spectroscopy (MilkoScan Mars™ analyzer) to determine the milk’s macro-nutrient composition, including the fat, protein and lactose contents (Fig. 1).
MeasurementThe 8 external probes read the temperature every 15 s. Data were recorded by 2 devices (Testo 176 and Testo 177) and downloaded to the corresponding software (JUMO PCA 3000). We performed 3 sets of pasteurization cycles with different amounts of cow’s milk.
We analysed the nutritional composition of 8 human milk samples before and after pasteurization. Each sample was preheated to 40 °C, following the directions of the milk analyzer manufacturer.
Data collectionWe used the quality control parameters for human milk pasteurizers proposed by Buffin et al. as reference. To verify correct and effective pasteurization, the analysis included the following parameters: (1) time from room temperature to 58 °C; (2) time from 58 °C to 62.5 °C; (3) duration of plateau; (4) time from 62.5 °C to 6 °C; (5) total duration of pasteurization cycle; and (6) highest temperature during plateau.
Data analysisWe collected data for the 3 pasteurization cycles, including the measurements obtained with the 8 temperature probes. For each cycle, we calculated the mean temperature in each of the 6 parameters with the corresponding 95% confidence interval. We used analysis of variance (ANOVA) to compare the 3 cycles and determine whether the treatment was homogenous regardless of the amount of cow’s milk pasteurized in each of them. We compared the mean values of macronutrients before and after pasteurization by means of the Student t test or, for nonparametric variables, the Mann–Whitney U test. Statistical significance was defined as a p-value of less than 0.05. We also calculated the magnitude of the variation in protein, fat and lactose contents as changes in percentage (% delta).
The analysis was performed with the software IBM SPSS Statistics version 22.
ResultsTable 1 shows the time and temperature measurements for the 3 pasteurization cycles. The last column presents the optimal values recommended by the European Milk Bank Association (EMBA) for Holder human milk pasteurization.7 There were no significant differences in 4 of the 7 parameters analyzed by comparing different volumes of milk: the time from 58 to 62.5 °C, duration of the plateau, highest temperature during the plateau and length of exposure to temperatures over 58 °C. All of these parameters met EMBA recommendations.
Mean time and temperature measurements (with 95% confidence intervals).
| Parameter | 120 mL | 60 mL | Mix volume | P | EMBAa |
|---|---|---|---|---|---|
| Time from room temperature to 58 °C (min) | 26.142 (24.82−27.45) | 20.06 (19.36−20.75) | 22.93 (21.31−24.55) | .000 | |
| Time from 58 to 62.5 °C (min) | 11.78 (10.98−12.59) | 11.10 (10.39−11.81) | 11.53 (10.38−12.67) | .466 | |
| Duration of plateau (min) | 31.60 (29.94−33.26) | 33.63 (32.36−34.89) | 32.90 (30.57−35.22) | .201 | 30−35 |
| Highest temperature (°C) | 63.58 (63.39−63.77) | 63.48 (63.31−63.65) | 63.32 (62.87−63.77) | .378 | 62.5−64 |
| Time from 62.5 to 6 °C (min) | 52 (49.30−54.69) | 40.38 (38.43−42.33) | 48.83 (46.87−50.79) | .000 | <60 |
| Time > 58 °C (min) | 45.60 (44.43−46.77) | 47.06 (46.45−47.67) | 45.78 (44.16−47.40) | .110 | <50 |
| Duration of cycle (min) | 120.9 (118.23−123.58) | 101.28 (99.22−103.34) | 116.09 (114−118.18) | .000 |
We found significant differences in the remaining 3 parameters (heating time from room temperature to 58 °C, cooling time from 62.5 °C to 6 °C and total duration of the pasteurization cycle), with a longer duration for the 120 mL cycle. Furthermore, the cooling time for the 120 mL cycle was also less than the 60 min recommended by the EMBA.
Pasteurization decreased the fat content of human milk (−6.2%), but there were no significant changes in the protein and lactose contents (−1.49% and no changes, respectively) (Table 2).
Macronutrient analysis before and after pasteurization of donor human milk (mean [95% CI]) and % delta).
| Nutrient | Before pasteurization | After pasteurization | P | % delta |
|---|---|---|---|---|
| Protein (g/100 mL) | 1.34 (1.33−1.34) | 1.32 (1.29−1.35) | .51 | −1.49% (0.7−3) |
| Fat (g/100 mL) | 3.06 (3.02−3.09) | 2.87 (2.81−2.94) | .00 | −6.2% (4.8−6.9) |
| Lactose (g/100 /mL) | 7.26 (7.22−7.30) | 7.29 (7.12−7.47) | .66 | No variation |
In our study, the recommended quality criteria for Holder pasteurization of human milk were met using a water-free pasteurizer independently of the milk volume treated in each cycle and the distribution of the bottles in the pasteurizer. The results showed a tendency for increased heating and cooling times in cycles with larger volumes, but these times did not exceed international quality standards.7
Holder pasteurization is the method used most commonly to treat human milk in milk banks worldwide.8 The technique has demonstrated a high microbiological safety, killing most bacteria and viruses with the exception of bacterial spores and hepatitis B virus with low-temperature heating (62.5 °C) over a long period of time (30 min).5 This treatment must be followed by a rapid cooling phase to prevent proliferation of residual bacteria. Concerns about the effects of heating and cooling duration and intensity on the quality of human milk, especially biologically active components, have motivated the industry to develop novel non-pasteurization techniques and improve current pasteurization methods by adjusting temperatures and shortening the duration of phases independently of the volume of milk being treated while ensuring homogeneity in different areas of the pasteurizer. Recent studies have shown that improved temperature control in critical phases of pasteurization results in better retention of major immune components in human milk.9,10 This has a special relevance when pasteurizing preterm DHM, which has more bioactive properties than term DHM,11 and given the wide variation in macronutrients between and within mothers depending on gestational age.12,13
The nutritional analysis revealed a similar lactose content and no significant decrease in protein content independently of the amount of pasteurized milk using volumes similar to those treated in clinical practice. The analysis of the fat content found a 6.2% decrease after pasteurization, which was similar to the decrease observed in other recent studies.14 At the PNU, pasteurization is focused on providing the best possible milk to each recipient, and very low birth weight infants are the most vulnerable patients and have special nutritional needs. Milk collected from the mothers of preterm infants has a different composition, not only based on the gestational age at delivery but also on the postnatal age of the donor’s child, with significant variations over time that extend to at least week 5 post delivery.15
Personalized preterm infant nutrition requires a direct and close relationship between the milk bank staff and donor mothers whose preterm infants are admitted to the neonatal unit. Many preterm infants, especially those under 28 weeks of gestation, are recipients of donor milk in the first hours post birth and, once the milk production of their own mothers exceeds their requirements, the mothers become donors themselves. The milk collected from any given donor is not pooled and is instead classified as colostrum, transitional or mature milk and also by range of gestational age at delivery: less than 28 weeks, 28–32 weeks, 32–37 weeks and term delivery. The volume of milk to be processed usually changes between pasteurization cycles depending on the type of milk being treated, with smaller volumes in bottles of colostrum from mothers that delivered earlier in the pregnancy.
Recent studies analysing preterm and term milk using “omics” technologies have evinced changes not only in macronutrients, but also, and much more significantly, in micronutrients, as the proteome, metabolome, microbiota and gene expression in the milk underwent dynamic changes during lactation that exhibit a natural adaptation to the individual needs of the infant.16 The effects of pasteurization on these components must be minimized using the best possible technique by controlling the quality of the pasteurizers currently in use in neonatal units.
In conclusion, Holder pasteurization of human milk performed with a water-free pasteurizer met the quality criteria recommended by the European Milk Bank Association independently of the quantity of milk pasteurized in each bottle, with significant changes in the fat content but not in the protein and lactose contents.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank M. Luisa García Serrano and Henar Muñoz Moyano, nurses of the Personalized Nutrition Unit, for their contribution and support in this study.
Please cite this article as: Caballero Martín S, Sánchez Gómez de Orgaz MC, Sánchez Luna M, Estudio de calidad de la pasteurización Holder de leche materna donada en una unidad de nutrición personalizada neonatal, Anales de Pediatría, 2022;96:294–299.