Approved drugs for attention deficit hyperactivity disorder (ADHD) in Spain are methylphenidate, lisdexamphetamine, atomoxetine and guanfacine. Due to adverse cardiovascular effects, mainly increased blood pressure and heart rate, its use in patients with known or undiagnosed heart disease may be controversial.
ObjectiveTo obtain a consensus document from the Spanish Society of Paediatric Cardiology and Congenital Heart Diseases (SECPCC) and experts from other Agencies and Societies as a guide for the paediatric cardiologist and physicians who treat children and adolescents with ADHD.
MethodAn analysis was performed on the bibliography and Clinical Practice Guidelines, technical data sheets approved by the Spanish Agency of Medicines and Health Devices, and the Spanish Ministry of Health Guidelines. A Working Group was formed, with a Coordinator, as well as members of the Clinical Cardiology Working Group and Arrhythmia Group of the SECPCC. This Group produced a preliminary document that was reviewed by a group of external experts (List 1) and a group of internal experts of the SECPCC (List 2) with a consensus being reached on the final document.
ResultsThe recommendations of the SECPCC and the group of experts are presented on cardiovascular evaluation prior to treatment in children and adolescents with unknown cardiovascular disease and with known cardiovascular disease.
The recommendations of the SECPCC and the group of experts are also presented on the use of medications for ADHD in children and adolescents with cardiological symptoms with no evidence of heart disease, congenital heart disease, cardiomyopathy, Marfan syndrome and other aortic diseases, hypertension, and arrhythmias.
Los fármacos aprobados para el trastorno por déficit de atención con hiperactividad (TDAH) en España son: metilfenidato, lisdexanfetamina, atomoxetina y guanfacina. Debido a los efectos adversos cardiovasculares que pueden producir, principalmente aumento de la tensión arterial y la frecuencia cardiaca, su uso en pacientes con cardiopatías conocidas o no diagnosticadas puede ser controvertido.
ObjetivoRealización de un documento de consenso de la Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas (SECPCC) y expertos de otras Agencias y Sociedades como instrumento para el cardiólogo infantil y los médicos que tratan niños y adolescentes con TDAH.
MetodologíaAnálisis de la bibliografía y Guías de Práctica Clínica, fichas técnicas aprobadas por la Agencia Española del Medicamento y Productos Sanitarios y Guía del Ministerio de Sanidad español. Formación de un Grupo de trabajo con un Coordinador, miembros de los grupos de trabajo de Cardiología Clínica y Arritmias de la SECPCC. Este Grupo realizó un documento que fue revisado por un grupo de expertos externos (Anexo 1) y un grupo de expertos internos de la SECPCC (Anexo 2) llegando a un consenso para la obtención del documento final.
ResultadosSe presentan las recomendaciones de la SECPCC y el grupo de expertos sobre la evaluación cardiovascular previa al tratamiento en niños y adolescentes sin enfermedad cardiovascular conocida y con enfermedad cardiovascular conocida. Se presentan las recomendaciones de la SECPCC y el grupo de expertos sobre el uso de medicamentos para el TDAH en niños y adolescentes con síntomas cardiológicos sin evidencia de cardiopatía, cardiopatías congénitas, miocardiopatías, síndrome de Marfan y otras aortopatías, hipertensión arterial y arritmias.
Attention-deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder usually with onset in childhood characterised by a persistent pattern of symptoms of inattention, hyperactivity and impulsivity (DSM-5).1 Broadly speaking, in children and adolescents pharmacological treatment is only recommended when cognitive-behavioural psychotherapy has failed or in case of moderate to severe symptoms.
The drugs authorised in Spain for treatment of ADHD are methylphenidate, lisdexamfetamine, atomoxetine and guanfacine. Due to the potential cardiovascular adverse effects of these drugs, mainly increases in blood pressure (BP) and heart rate, their use in patients with known or undiagnosed congenital heart defects (CHDs) is controversial.
ObjectiveTo develop a consensus document of the Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas (Spanish Association of Paediatric Cardiology and Congenital Heart Disease, SECPCC) in collaboration with experts from other societies and agencies useful to paediatric cardiologists and physicians managing children and adolescents with ADHD.
MethodologyAnalysis of the scientific literature and international clinical practice guidelines, summary of product characteristics of drugs authorised by the Agencia Española del Medicamento y Productos Sanitarios (Spanish Agency of Medicines and Medical Devices) and the Guideline of the Spanish Ministry of Health.
To this end, a working group was formed consisting of a coordinator, members of the Working Group on Clinical Cardiology and the Group on Arrhythmias of the SECPCC with relevant clinical experience on the subject. This group developed an initial version of the document that was revised by an external group of experts (Appendix A) and an internal group of experts of the SECPCC (Appendix B), eventually reaching a consensus on the final document.
Recommendations of the Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas on the cardiovascular evaluation preceding treatmentIn children and adolescents with unknown cardiovascular diseaseBased on the current literature,2 and in agreement with the main international guidelines,3–8 the SECPCC recommends performing a cardiovascular evaluation before initiating treatment for ADHD in children and adolescents with unknown cardiovascular disease by means of a history taking and physical examination. Routine performance of an electrocardiogram (ECG) is not recommended, but should be reserved for selected cases as described below.
History taking: emphasis should be placed on the identification of known heart diseases and the presence of warning signs such as syncope suggestive of a cardiac origin (Table 1) or from a non-vasovagal cause, chest pain suggestive of a cardiac origin, palpitations or shortness of breath on exertion. It is also important to ask about the history of sudden death in relatives aged less than 40 years or the presence of heart disease in the family, including hypertrophic cardiomyopathy, long QT syndrome or other channelopathies, Wolff-Parkinson-White syndrome, bicuspid aortic valve, coarctation of the aorta, arterial hypertension (HTN) at an early age or renal disease. In case of warning signs or a reasonable suspicion of heart disease, the patient should be referred to a cardiologist before initiating pharmacological treatment.
Syncope suggesting a cardiac origin.
| Presence of structural heart disease |
| Family history of SCD |
| Syncope on exertion, associated with emotional states or in supine position |
| Palpitations or chest pain of sudden onset followed by syncope |
| Presence of organic murmur |
| Abnormal ECG |
SCD, sudden cardiac death.
Physical examination: It should include measurement of the weight and height of the patient. Particular attention should be paid to the detection of murmurs during auscultation and the identification of features of Marfan syndrome, such as tall final height relative to the predicted target height, tall and slender build, pectus carinatum or excavatum, long and narrow face, long and slender fingers, limited extension of the elbow or scoliosis, among others. In case of detection of a murmur or features of Marfan syndrome, the patient must be referred to a cardiologist prior to initiation of pharmacotherapy. Since several studies have found evidence of mild increases in heart rate and BP (both systolic and diastolic) in association with the use of stimulant and nonstimulant drugs for treatment of ADHD,9 we recommend including measurement of the heart rate and BP in the initial assessment prior to initiation of pharmacotherapy, with subsequent monitoring of both variables every 3–6 months.
Blood pressure: we recommend adherence to the protocol for measurement of BP proposed by the European Society of Hypertension,10 using a cuff of appropriate size for the age of the patient, and calculating the percentiles of BP based on the height of the patient. If the initial BP value is high, the measurement should be repeated at least twice. If it remains high (> 95th percentile), an evaluation of HTN should be performed by means of ambulatory blood pressure monitoring (ABPM), and if HTN is confirmed, this should be followed by an investigation of its aetiology and an evaluation of potential damage in target organs. If a high BP is detected in patients already undergoing pharmacological treatment for ADHD, the dose of medication could be reduced or medication suspended before starting the evaluation of HTN. Once a diagnosis is established, the management of the patient can include a combination of antihypertensive drugs and pharmacotherapy for ADHD with guanfacine, which is associated with hypotension, or the dosage of ADHD medication can be reduced or the medication discontinued altogether.
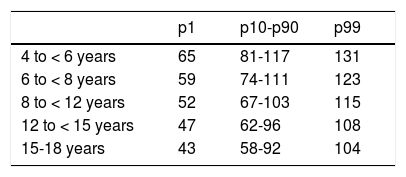
Heart rate: in case of detection of a heart rate that is persistently above the 99th percentile (Table 2), potential psychological and medical causes should be explored, and an ECG performed to confirm that the patient has sinus tachycardia and not another form of arrhythmia. It is important to consider that patients with ADHD frequently experience anxiety symptoms that may in turn be associated with increased heart rate. It is possible, although rare, that the sinus tachycardia is severe enough to require adjustment or discontinuation of pharmacological treatment.
Heart rate (bpm) by age group and percentile range.
| p1 | p10-p90 | p99 | |
|---|---|---|---|
| 4 to < 6 years | 65 | 81-117 | 131 |
| 6 to < 8 years | 59 | 74-111 | 123 |
| 8 to < 12 years | 52 | 67-103 | 115 |
| 12 to < 15 years | 47 | 62-96 | 108 |
| 15-18 years | 43 | 58-92 | 104 |
bpm, beats per minute.
Adapted from Fleming et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet 2011;377:1011.
Electrocardiogram: performance of an ECG is not recommended as part of the routine cardiovascular assessment prior to initiating pharmacotherapy. In case of treatment with guanfacine and atomoxetine combined with other drugs that may prolong the QT interval (such as escitalopram or fluoxetine), or of treatment combining methylphenidate and risperidone,11 monitoring of the QTc interval is advisable. An ECG should be performed if the patient requires referral to a cardiologist due to abnormal findings in the cardiovascular assessment, symptoms suggesting cardiovascular origin or a family history of sudden death.
In children and adolescents with known heart diseaseIf the patient has a known cardiac disease, we recommend consultation with a paediatric cardiologist prior to initiating pharmacotherapy.
The cardiologist and ADHD specialist should discuss the appropriate approach for pharmacotherapy with the family, engaging in shared decision-making after providing the family with adequate information and taking into account the risks and benefits of treatment.
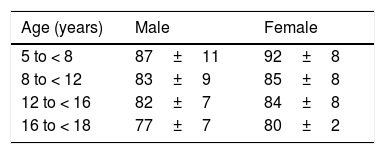
We recommend measurement of BP and heart rate before initiation of treatment with additional measurements every 3–6 months (Table 2). In patients with CHDs and a circulatory physiology that could be compromised by tachycardia or increased BP, the BP and heart rate should be monitored every 1 or 2 months. In case of detection of tachycardia or a history indicative of arrhythmia, the patient should be evaluated by means of 24-h Holter monitoring (Table 3).
Mean heart rate (bpm) and standard deviation in 24-h Holter monitoring.
| Age (years) | Male | Female |
|---|---|---|
| 5 to < 8 | 87±11 | 92±8 |
| 8 to < 12 | 83±9 | 85±8 |
| 12 to < 16 | 82±7 | 84±8 |
| 16 to < 18 | 77±7 | 80±2 |
bpm, beats per minute.
Adapted from Krasemann et al. Changes of the corrected QT interval in healthy boys and girls over day and night. Eur Heart J. 2009;30:202–208.
- •
Children or adolescents with a diagnosis of neurocardiogenic syncope or symptoms suggestive of orthostatic hypotension can start pharmacotherapy for ADHD, avoiding the use of guanfacine due to its hypotensive effect.
- •
Children or adolescents with a diagnosis of nonspecific chest pain or palpitations with a normal cardiological evaluation (ECG, echocardiogram, Holter ECG or cardiac stress test, as applicable), can start pharmacotherapy for ADHD using stimulant or nonstimulant drugs.
- •
Children or adolescents with a family history of sudden death in the absence of evidence of inherited heart conditions, and with a normal cardiological evaluation (ECG, echocardiogram, Holter ECG or cardiac stress test, as applicable) and negative results of genetic testing or in who genetic testing is not indicated, can start pharmacotherapy for ADHD.
- •
In children or adolescents with a family history of sudden death, a normal cardiological evaluation (ECG, echocardiogram, Holter ECG or cardiac stress test, as applicable) and positive results of genetic testing but a negative phenotype at the time of the evaluation, decisions regarding pharmacotherapy should be individualised.
The following patients may receive medication for ADHD:
- •
Patients with mild and haemodynamically insignificant CHDs that had not undergone corrective surgery and do not require treatment for the heart disease.
- •
Patients with simple or complex CHDs that have undergone successful corrective surgery, without residual lesions and not receiving treatment for the heart disease.
The use of pharmacological treatment for ADHD should be individualised in the following cases:
- •
Patients particularly sensitive to an increase in afterload due to an increase in BP secondary to stimulant therapy, such as patients with ventriculoarterial valve or systemic atrioventricular valve regurgitation.
- •
Patients particularly sensitive to a decrease in afterload due to a potential decrease in BP secondary to treatment with guanfacine, such as patients with systemic ventricular outflow obstruction.
- •
Patients particularly sensitive to potential tachycardias secondary to stimulant therapy, such as those with diastolic dysfunction or mitral stenosis in whom a shortening of the diastole should be avoided, or patients with systolic dysfunction in whom an increase in myocardial oxygen consumption secondary to tachycardia should be avoided.
- •
Patients at risk of coronary failure: patients with Kawasaki disease, that have undergone coronary reimplantation (arterial switch procedure, Ross procedure, anomalous left coronary artery from the pulmonary artery), with anomalous origin or paths of coronary arteries, vasculitis, or early atherosclerosis of the coronary arteries. In these cases, it is important to ensure that the coronary flow reserve is sufficient in case of a potential increase in myocardial oxygen consumption secondary to the use of sympathomimetic drugs.
- •
In all cases, we recommend clinical monitoring of the patient after treatment initiation, assessing changes in BP, heart rate and ECG features and the haemodynamic impact of these changes after initiating treatment and any increase in dosage. The frequency of these assessments should be established on a case-by-case basis.
- •
Dose adjustments or discontinuation of ADHD medication should be considered in case of a haemodynamic response that can have a negative impact on the patient’s heart condition.
We recommend against initiating pharmacological treatment in:
- •
Patients with haemodynamically unstable CHD, before the haemodynamically or clinically significant lesion is corrected or treated.
- •
Patients with CHD and residual HTN. Adequate management and control of BP must precede initiation of pharmacological treatment for ADHD.
The risks and benefits of ADHD treatment should be evaluated on a case-by-case basis in collaboration with the ADHD specialist (neurologist, psychiatrist or paediatrician).
Initiation of pharmacotherapy is not recommended in patients with haemodynamically unstable cardiomyopathy.
We recommend clinical monitoring of the patient after initiation of treatment, assessing changes in BP, heart rate and ECG features as well as the impact of these changes in the haemodynamic status of the patient. These variables should be assessed on treatment initiation and after each dose increase, if applicable.
Dose adjustment or discontinuation of ADHD medication should be considered in patients exhibiting haemodynamic changes that may have a negative impact on the cardiomyopathy.
Marfan syndrome and other aortopathiesPatients with a normal aortic root diameter, with or without ongoing treatment, can start pharmacotherapy for ADHD with monitoring of BP and heart rate every month or 2 months and whenever the dose is increased.
In patients with a dilated aorta undergoing treatment with beta-blockers or angiotensin II receptor blockers, it is particularly important to be prudent in the decision to initiate ADHD medication, as an increase in heart rate or BP could be highly detrimental. In any case, the drug with the least sympathomimetic activity available should be selected, for instance, guanfacine. Another important aspect to consider is that since these patients are receiving hypotensive treatment, they may not tolerate further increases in BP.
In case of HTN, ADHD treatment should be suspended and BP controlled with pharmacotherapy before gradually reintroducing ADHD medication.
In case of concomitant mitral valve regurgitation, treatment should also adhere to the recommendations given in the CHD section of this guideline.
High blood pressureBlood pressure should be measured following the technical recommendations and reference values of the European Society of Hypertension.12
The BP should be measured before initiating and during treatment with ADHD drugs at intervals to be determined on a case-by-case basis considering everything that has been discussed to this point. It is important to use a cuff size appropriate for the age of the patients and to calculate the BP percentiles based on height.12,13
- •
If the BP is below the 95th percentile, ADHD pharmacotherapy can be initiated/continued.
- •
If the BP is above the 95th percentile, it must be measured 2 more times after intervals of 10min of rest; if the BP continues to be high, the dose should be reduced or the ADHD medication suspended temporarily, with subsequent monitoring of BP.
- a
If the BP is below the 95th percentile in the next check up, treatment can continue or resume.
- b
If the BP is above the 95th percentile in the next check up, the patient should be referred to a paediatric nephrologist for performance of an evaluation including a 24-h ABPM. If the findings of ABPM are abnormal, we also recommend performance of an ECG and an echocardiographic evaluation. A second ABPM should be performed, and in case of abnormal findings, HTN should be diagnosed and the required treatment initiated.. If the second ABPM does not confirm the diagnosis of HTN, ADHD treatment can be initiated.
- ○
Once HTN has been diagnosed and relevant treatment initiated, if control of BP is achieved with sustained values below the 95th percentile, it is possible to initiate or resume pharmacotherapy for ADHD.
- ○
It is important to recommend nonpharmacological lifestyle interventions in all patients with high blood pressure10: physical activity, dietary recommendations (increased intake of fruit, vegetables and legumes, choosing low-fat dairy products, decreased intake of salt and sugars), weight loss as needed, and stress reduction.
- ○
In children with ADHD, the prevalence of anxiety is much higher compared to the general population, so any measure aimed at reducing stress should be considered, as well as specific treatment for anxiety, if the patient requires it. Anxiety is associated to increase both BP and heart rate.
- ○
We recommend against pharmacological treatment of ADHD (with stimulants or atomoxetine) in patients with moderate or severe HTN.5,14
- ○
In case of elevation of BP with psychostimulant drugs (methylphenidate and lisdexamfetamine), consider the addition of a beta-blocker on a case-by-case basis.
- ○
It is important to take into account that stimulants may reduce the effectiveness of antihypertensive drugs, and caution should be exerted when they are administered along with drugs that also increase BP.
- ○
In case of moderate HTN, treatment with guanfacine may be considered. It is important to remember that the dose of guanfacine should be tapered off before discontinuation to minimise the risk of rebound HTN.
- ○
If during treatment with guanfacine the patient develops sustained orthostatic hypotension or episodes of syncope, the dose should be reduced or the ADHD medication switched to a different drug.
- ○
- a
Treatment for ADHD with stimulant or nonstimulant drugs can be initiated in:
- •
Patients with incidental findings in ECG that are frequent in childhood and adolescence and are not pathological: incomplete right bundle branch block, respiratory sinus arrhythmia or wandering atrial pacemaker.
- •
Patients with incidental finding in ECG of complete right bundle branch block or left anterior or posterior hemiblock, once structural heart disease has been ruled out.
- •
Asymptomatic patients with incidental detection of infrequent (< 60/h) monomorphic premature supraventricular contractions after ruling out relevant associated disease. We recommend performance of 24-h Holter monitoring after treatment initiation to assess for changes in the pattern of arrhythmia relative to baseline.
- •
Patients with Brugada syndrome, as the highest risk in these patients corresponds to situation with increased vagal tone.
- •
Patients with a history of supraventricular tachycardia currently not receiving antiarrhythmic drugs. In case of persistence or recurrence of tachycardia, consider the possibility of cardiac ablation for curative treatment.
- •
Asymptomatic patients with infrequent (< 60/h) monomorphic premature ventricular contractions (PVCs) that are not complex, without underlying structural heart disease and with evidence of a decrease or disappearance of PVCs on exertion in the cardiac stress test. We recommend performance of 24-h Holter monitoring after treatment initiation to assess for changes in the pattern of arrhythmia relative to baseline.
- •
Asymptomatic patients with incidental finding of preexcitation in the ECG and evidence of disappearance of impulse conduction through the accessory pathway with development of a high heart rate in the cardiac stress test or 24-h Holter monitoring, in who it is not necessary to ablate the accessory pathway before initiating treatment.
- •
Asymptomatic patients with incidental finding of first degree or second degree (Mobitz I) atrioventricular block, avoiding the use of guanfacine.
In the following cases, it is necessary to consult with a specialist in arrhythmias and individualise treatment:
- •
Patients with catecholaminergic polymorphic ventricular tachycardia. This is an infrequent disease with catecholamine-dependent features, so stimulant therapy is not recommended in these patients.
- •
Asymptomatic patients with frequent (> 60/h) monomorphic PVCs, polymorphic PVCs or complex PVCs, or with underlying structural heart disease.
- •
Asymptomatic patients with incidental finding of preexcitation on ECG and no evidence of disappearance of impulse conduction through the accessory pathway with development of a high heart rate in the cardiac stress test or 24-h Holter monitoring.
- •
Previous episode of aborted sudden cardiac death.
- •
Previous history of arrhythmia requiring cardiopulmonary resuscitation, cardioversion, defibrillation or a pacemaker.
- •
History de arrythmia associated with sudden death.
The authors have no conflicts of interest to declare.
Agencia Española del Medicamento y Productos Sanitarios (Spanish Agency of Medicines and Medical Devices). Almudena López Fando, Division of Pharmacoepidemiology and Pharmacosurveillance. Department of Medicinal Products for Human Use.
Sociedad Española de Cardiología (Spanish Cardiology Association). Inmaculada Sánchez, Paediatric Cardiology, Hospital Ramón y Cajal Madrid.
Sociedad Española de Psiquiatría (Spanish Psychiatric Association). José Antonio Ramos Quiroga, coordinator of the Programme on Attention-Deficit Hyperactivity Disorder and coordinator of the Psychiatric Emergency Department, Hospital Universitari Vall d’Hebron, Barcelona.
Sociedad Española de Farmacología Clínica (Spanish Society of Clinical Pharmacology). Belén Ruiz Antorán, Expert Group of the European Medicines Agency, Department of Clinical Pharmacology, Hospital Puerta de Hierro, Madrid.
Sociedad de Psiquiatría Infantil de la Asociación Española de Pediatría. Azucena Díez Suárez, Unidad de Psiquiatría Infantil y Adolescente, Clínica Universidad de Navarra.
Asociación Española de Pediatría (Spanish Association of Pediatrics), Working Group on Evidence-Based Paediatrics. Carlos Ochoa Sangrador, Hospital Virgen de la Concha, Zamora.
Sociedad Española de Neurología Pediátrica (Spanish Society of Paediatric Neurology). Francisco Javier López Pisón, Paediatric Neurology, Hospital Miguel Servet, Zaragoza.
Asociación Española de Pediatría de Atención Primaria (Spanish Association of Primary Care Paediatrics, AEPap). José Miguel García Cruz, coordinator of the Group on ADHD and Psychoeducational Development of the AEPap.
Sociedad Española de Pediatría Extrahospitalaria y Atención Primaria (Spanish Association of Outpatient and Primary Care Paediatrics). Adrián García Ron, Paediatric Neurology, Hospital Clínico San Carlos, Madrid.
Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas (Spanish Association of Paediatric Cardiology and Congenital Heart Disease). Miguel Ángel Granados Ruiz, Paediatric Cardiology, Hospital 12 Octubre, Madrid.
Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas (Spanish Associatio of Paediatric Cardiology and Congenital Heart Disease). María del Mar Rodríguez Vázquez del Rey, Paediatric Cardiology, Hospital Virgen de las Nieves, Granada.
Sociedad Española de Cardiología Pediátrica y Cardiopatías Congénitas (Spanish Association of Paediatric Cardiology and Congenital Heart Disease). Elena Montañés Delmas, Paediatric Cardiology, Hospital 12 Octubre, Madrid.
Please cite this article as: Picarzo JP-L, Malfaz FC, Marcos DC, Hernández RC, Soria TF, García BM. Recomendaciones de la Sociedad Española de Cardiologia Pediatrica y Cardiopatias Congenitas en relacion al uso de medicamentos en el trastorno por déficit de atención e hiperactividad en niños y adolescentes con cardiopatias conocidas y en la población pediatrica general, posicionamiento de la Asociación Española de Pediatria. An Pediatr (Barc). 2019. https://doi.org/10.1016/j.anpedi.2019.09.002