In pregnant women who have been exposed to tuberculosis (TB), primary isoniazid prophylaxis is only recommended in cases of immunosuppression, chronic medical conditions or obstetric risk factors, and close and sustained contact with a patient with infectious TB. Isoniazid prophylaxis for latent tuberculosis infection (LTBI) is recommended in women who have close contact with an infectious TB patient or have risk factors for progression to active disease. Otherwise, it should be delayed until at least three weeks after delivery. Treatment of TB disease during pregnancy is the same as for the general adult population. Infants born to mothers with disseminated or extrapulmonary TB in pregnancy, with active TB at delivery, or with postnatal exposure to TB, should undergo a complete diagnostic evaluation. Primary isoniazid prophylaxis for at least 12 weeks is recommended for those with negative diagnostic tests and no evidence of disease. Repeated negative diagnostic tests are mandatory before interrupting prophylaxis. Isoniazid for 9 months is recommended in LTBI. Treatment of neonatal TB disease is similar to that of older children, but should be maintained for at least 9 months. Respiratory isolation is recommended in congenital TB, and in postnatal TB with positive gastric or bronchial aspirate acid-fast smears. Separation of mother and infant is only necessary when the mother has received treatment for less than 2 weeks, is sputum smear-positive, or has drug-resistant TB. Breastfeeding is not contraindicated, and in case of mother–infant separation expressed breast milk feeding is recommended.
En la embarazada expuesta a tuberculosis (TB) no se recomienda profilaxis primaria con isoniazida salvo en gestantes inmunodeprimidas, con enfermedades crónicas o factores de riesgo obstétrico y antecedente de contacto íntimo mantenido con un enfermo bacilífero. En la infección tuberculosa latente (ITBL) se iniciará profilaxis con isoniazida si existe contacto reciente con TB o factores de riesgo de progresión a TB activa. En caso contrario, se retrasará hasta al menos 3 semanas después del parto. El tratamiento de la enfermedad tuberculosa es el mismo que fuera de la gestación. Los recién nacidos de madres con historia gestacional de TB diseminada o extrapulmonar, con TB activa en el parto o con contacto TB posnatal conocido, asintomáticos y con pruebas diagnósticas negativas, deben recibir profilaxis primaria con isoniazida durante al menos 12 semanas. Transcurrido ese tiempo se repiten los test diagnósticos, y si son negativos, se interrumpe la profilaxis. En la ITBL, se administrará isoniazida durante 9 meses. En la enfermedad tuberculosa, el tratamiento es el mismo que en el niño mayor pero mantenido al menos 9 meses. Se recomienda aislamiento respiratorio en la TB congénita y en la TB posnatal con baciloscopia positiva en jugo gástrico o aspirado bronquial. La separación madre-hijo solo está indicada en madres que han recibido tratamiento durante menos de 2 semanas, presentan baciloscopia positiva o tienen TB resistente. La lactancia materna no está contraindicada y en las situaciones de separación la madre puede extraerse la leche para que sea administrada en biberón al recién nacido.
It is recommended that prophylaxis is withheld and that the tuberculin skin test (TST) and/or interferon gamma release assay (IGRA) is repeated after 8–12 weeks, as initial tests in very recent infections may yield negative results. The possibility of initiating primary prophylaxis with isoniazid prior to the repetition of the diagnostic tests will only be considered in pregnant women that have had close and sustained contact with a case of active infectious TB if they are immunosuppressed or have a chronic medical condition or obstetric risk factors. In pregnant women with severe immunosuppression, a six-month course of prophylaxis should be administered even if the repeat TST/IGRA results are negative, as the sensitivity of these tests is lower in immunosuppressed individuals.
Latent tuberculosis infection- –
Latent tuberculosis infection (LTBI) with prophylaxis treatment before pregnancy. If prophylactic treatment of LTBI is initiated and then the patient gets pregnant, the prescribed course must be continued to its completion.1
- –
LTBI with neither recent TB contacts or risk factors for progression to active tuberculosis (TB). If the investigation of contacts fails to document a recent contact with a case of TB and the pregnant patient does not have risk factors for progression to active disease (infection by human immunodeficiency virus, immunosuppression, intravenous drug use, crowded living conditions or social exclusion), it is recommended that prophylaxis with isoniazid is postponed to at least three weeks post partum to minimise foetal risk and the risk of gestational or postpartum hepatitis in the mother.2,3 Breastfed children of mothers treated with isoniazid should receive supplemental pyridoxine (1–2mg/kg/day).
- –
LTBI with recent TB contacts or risk factors for progression to active TB. Prophylaxis should be initiated as soon as possible, regardless of the weeks of gestation (even in the first trimester).4 The indicated drug is isoniazid (5–10mg/kg/day as a single dose; maximum dose 300mg) for 6 months. If the tablet formulation does not include vitamin B6, a pyridoxine supplement (10–50mg/day) should be added to prevent the development of peripheral neuropathies. Isoniazid can cause hepatotoxicity through cytolysis during pregnancy and the postpartum period, and there are published cases of maternal acute liver failure and death.5 Thus, it is advisable to verify that the pregnant patient has normal serum transaminase levels before initiating treatment, and these levels must be determined in all patients 2 weeks after treatment implementation and also at 1, 2, 4 and 6 months.
In cases of severe intolerance or resistance to isoniazid, rifampicin (10mg/kg/day; maximum dose 600mg) will be administered for four months. One of its main adverse effects is hepatitis with cholestasis, so liver enzymes should be monitored as they are monitored in patients treated with isoniazid.
Pregnant women that are going to be treated with tuberculostatic drugs should be asked about their history of chronic liver disease and their consumption of alcohol and other hepatotoxic agents, as the frequency and severity of liver toxicity increase when these factors are present.2,6
Tuberculosis diseaseAntituberculosis therapy should be initiated when the presence of Mycobacterium tuberculosis is confirmed by polymerase chain reaction or culture from any biological sample or there is evidence of granulomatous inflammation in a tissue sample of a patient with compatible symptoms. However, in pregnant women with clinical or radiological features highly suggestive of TB or with suspected severe TB, such as meningitis or miliary dissemination, urgent treatment should be initiated without waiting for microbiological or histopathological results.
- –
First-line treatment. Treatment of tuberculosis in pregnant women is the same as the treatment recommended outside of pregnancy. It consists of the combination of isoniazid (5–10mg/kg/day; maximum dose 300mg), rifampicin (10mg/kg/day; maximum dose 600mg), pyrazinamide (25–30mg/kg/day; maximum dose 2.000mg) and ethambutol (15–20mg/kg/day; maximum dose 1600mg) for two months, followed by isoniazid with rifampicin for four more months, for a total course lasting six months. Pyrazinamide was not used for many years due to a lack of data on its teratogenicity. However, no side effects have been described on the foetuses of treated women, so at present most scientific associations recommend it for first-line treatment.7 Ethambutol can be suspended once it has been confirmed that the isolate is susceptible to the three other first-line drugs.
The duration of treatment in cases of tuberculous meningitis will be of 12 months. In cases of tuberculous meningitis and pericarditis, corticosteroids will also be administered in the early weeks of treatment for their anti-inflammatory action. The recommended dosage is 0.5–1mg/kg/day of methylprednisolone for one month, with progressive tapering down to completion of treatment at 2 months. One of the main adverse effects of treatment is liver toxicity caused by isoniazid, pyrazinamide and rifampicin. If the patient develops clinical manifestations of hepatitis and transaminases are elevated up to 3 times their normal value or more, or, in the absence of hepatitis symptoms, when transaminases are elevated above five times their normal value in laboratory tests, treatment will be suspended until the levels normalise. The first-line drugs will then be reintroduced gradually.2,6 If the problem recurs, the patient will be treated with non-hepatotoxic drugs.
- –
Second-line treatment. Used in pregnant women when there is resistance or intolerance to first-line drugs; consultation with a TB expert is advisable. It is recommended that treatment be initiated starting from the second trimester of gestation, although it may be initiated during the first trimester in cases of severe disease. Streptomycin, amikacin, kanamycin and capreomycin (category D) are contraindicated, as they can cause congenital deafness. The use of ethionamide-prothionamide must be avoided, as there is little experience with them during pregnancy and there is a risk of teratogenicity, as should be the use of quinolones (moxifloxacin, levofloxacin), since there have been reports of adverse effects on foetal bone formation. Cycloserine and 4-aminosalicylic acid (category C) could be used in the absence of alternative treatments, although no data are available on their safety in pregnancy and in the foetus.2,7,8
It is recommended that pregnant women receiving antiretroviral therapy be treated with the first-line antituberculosis treatment described above, as long as the patient is treated with two nucleoside analogues in combination with efavirenz (this drug must be avoided in the first 8 weeks of gestation),9 raltegravir or nevirapine.8–10 If these drug combinations cannot be used and the patient needs a protease inhibitor, rifampicin can be replaced by rifabutin, which has fewer interactions with protease inhibitors, adjusting the dose accordingly.11,12 Therapeutic drug monitoring of antiretrovirals should be considered in these patients.
Follow-upTreatment must be strictly monitored to ensure adherence and efficacy and detect side effects from the drugs. In most patients follow-up at 15 days and 1, 2, 4, and 6 months of treatment suffices, performing radiographic and microbiological tests whenever necessary.
BreastfeedingExcept in cases of tuberculous mastitis, tuberculosis disease during pregnancy or the postpartum period does not contraindicate breastfeeding as long as the mother has received two weeks of appropriate treatment, the disease is not active and infectious, the strain is susceptible to first-line treatment drugs and the newborn is receiving prophylactic treatment with antituberculosis drugs. When any of these conditions are not met, the mother can express her breast milk to administer to the newborn from a bottle. M. tuberculosis is not transmitted from breast milk and the small concentrations of drugs in the milk do not cause significant toxicity in infants. However, second-line drugs must be used with caution, due to lack of data and their potential toxicity. Breastfed children of mothers treated with isoniazid, cycloserine or ethionamide-prothionamide should receive pyridoxine supplementation (1–2mg/kg/day).2,7,12
Prophylaxis and treatment of neonatal tuberculosisProphylaxis of LTBI and treatment of active TB in neonates are based on first-line antituberculosis drugs (Table 1). The doses are those recommended for infants older than 3 months of age, although there are no pharmacological data validating their safety or efficacy at younger ages.13 Thus, close clinical monitoring of these patients is essential to assess the effectiveness and potential toxicity of the treatment.
Available formulations, routes of administration and recommended doses of first-line antituberculosis drugs in Spain.
| Drug | Dosage form | Route | Recommended dose (range), mg/kg/day | |
|---|---|---|---|---|
| Isoniazida,b | Cemidón 50 B6© | Tablet, 50/15mg | Oral | 10 (10–15) |
| Cemidón 150 B6© | Tablet, 150/25mg | |||
| Cemidón 300 B6© | Tablet, 300/50mg | |||
| Cemidón (slow IV/IM)© | Ampoule, 300mg in 5mL | IV/IM | ||
| Rifampicin | Rifaldin 300©/Rimactan 300© | Capsule, 300mg | Oral | 15 (10–20) |
| Rifaldin 600© | Tablet, 600mg | |||
| Rifaldin suspensión© | Syrup, 20mg/ml | |||
| Rifaldin 600 IV© | Ampoule, 600mg in 10mL | IV | ||
| Pyrazinamide | Pirazinamida Prodes© | Tablet, 250mg | Oral | 30 (20–35) |
| Ethambutol | Myambutol© | Tablet, 400mg | Oral | 15 (15–20) |
| Amikacin | (Various commercial brands and dosage forms) | IV/IM | ||
The problem is compounded by the lack of commercial formulations appropriate for infants in Spain, with the exception of rifampicin oral suspension. Some hospital pharmacies compound preparations that are appropriate for neonates. Parents or caregivers must be educated on the preparation and administration of the treatment, and on tolerance problems or adverse effects that they need to watch for. Medication must be administered as a single daily dose and on an empty stomach to facilitate its absorption. This may prove difficult in neonates, and it is recommended that medication is given just before breastfeeding, and if possible, that the child continues to fast for a few minutes after its administration.14
Adherence to antituberculosis treatment is essential to guarantee therapeutic success. The World Health Organization and the Centers for Disease Control and Prevention recommend directly observed therapy for all paediatric patients.15 In Spain, this option is not available in most facilities, although it may be necessary in select cases, especially those involving high social risk patients or drug-resistant strains.
Exposure to tuberculosisNewborns of a mother with a history of disseminated or extrapulmonary disease during pregnancy, active TB at the time of delivery or with known postnatal exposure, that are asymptomatic, and in whom all initial diagnostic tests are negative, should receive primary prophylaxis with isoniazid. Withholding prophylaxis is an option if the mother has a history of uncomplicated pleural or pulmonary TB during pregnancy, is receiving appropriate treatment with good adherence, has had at least one negative sputum culture, did not have active infectious TB at the time of delivery, and a meticulous investigation of contacts has ruled out the presence of TB in the household. In these cases, close clinical monitoring of the child is recommended, as well as performing a TST and IGRA at birth and every three to four months up to 1 year of age. In uncertain cases or cases that do not match all of the above criteria, all the initial diagnostic tests will be performed, and if they are negative and the child is asymptomatic, prophylactic treatment will be initiated.
Prophylaxis with isoniazid must last a minimum of 12 weeks.16 At 12 weeks, the TST and the IGRA are repeated, and if they are negative treatment is discontinued, although the patient must be closely monitored and the diagnostic tests repeated at 6 and 12 months of life, given the lower sensitivity of these tests in the neonatal period. If the strain of the index case is resistant to isoniazid, rifampicin (10–15mg/kg/day) will be used for prophylaxis for a minimum of 12 weeks.16 If the isolate is resistant to isoniazid and rifampicin (multidrug resistance), the patient may receive prophylactic treatment with alternative drugs (such as fluoroquinolones) or be left untreated under close clinical monitoring with repetition of the diagnostic tests at 1½, 3, 6 and 12 months.
In newborns with a high-risk contact (mother with severe or disseminated TB close to the time of delivery or in the postpartum period, or very close or prolonged postnatal contact with a highly infectious TB case of active disease), prophylaxis can be extended until diagnostic tests yield negative results at 6 months of life. In preterm, malnourished or immunosuppressed infants, in whom the sensitivity of the TST and IGRAs is lower, there is the option of administering nine-month courses of prophylaxis, as is done for LBTI, even when all test results are negative, or of extending treatment to at least three months after the cultures of the index case become negative.
Latent tuberculosis infectionNeonates with positive TST or IGRA results that have no symptoms and with negative results in all first- and second-tier diagnostic tests should receive secondary prophylaxis with isoniazid for nine months.16 A six-month course of rifampicin (10–15mg/kg/day) is recommended in cases of confirmed isoniazid resistance.16 A three-month course of isoniazid and rifampicin in combination is not recommended due to the risk of hepatotoxicity and the lack of data for very young children, although it facilitates adherence and may be useful in select cases, especially if isoniazid resistance is suspected in the index case.
Routine supplementation with vitamin B6 (1–2mg/kg/day) is recommended in neonates treated with isoniazid, and in Spain both are usually delivered in a single tablet of isoniazid-B6 (50–15mg or 150–25mg).
Consultation with an expert is recommended in infants with LTBI by a multidrug-resistant strain. Dual therapy with pyrazinamide and ethambutol or other two drugs with confirmed activity against the strain can be used in directly observed therapy for nine to twelve months, although the most adequate drug combination, the duration of treatment and its effectiveness have not been established.
Tuberculosis diseaseTreatment of neonatal tuberculosis disease, as in adults and older children, consists of an initial phase and a continuation phase. The recommendation for the initial phase is a combination of four first-line tuberculostatic drugs for two months, which must always include isoniazid, rifampicin and pyrazinamide and with amikacin or ethambutol as the fourth drug.17 The fourth drug should be discontinued once the susceptibility of the isolate of the patient or the index case to the other three first-line drugs has been confirmed. Ethambutol is usually preferred because it can be administered orally and it has a lower toxicity, as optic neuritis is rare if the dose does not exceed recommendations. However, amikacin is preferable for initial treatment in cases of severe or disseminated disease or with central nervous system involvement.
In the continuation phase, after ascertaining the improvement of symptoms, the patient is treated with a combination of isoniazid and rifampicin at the usual doses for a duration established based on the type of disease, initial severity, and the patient's response to treatment. A total course of at least 9 months is recommended for neonatal TB,17,18 although it should be extended to a minimum of 12 months for disseminated or meningeal forms of disease, and to 18–24 months for multidrug-resistant TB. Intermittent therapy with doses given 2 or 3 times a week is not recommended.
In tuberculous disease in which the causative agent is resistant to first-line drugs, the treatment regimen is chosen based on the same criteria as those applied to older children. However, the lack of specific pharmacological data for many of the second-line antituberculosis drugs in the neonatal age group makes the treatment even more complicated, so we recommend that an expert be consulted in every case.
Corticosteroid therapy is clearly indicated in cases with central nervous involvement, miliary TB and in the presence of bronchial compression due to adenopathy. It can also be useful in extrapulmonary forms of disease with a significant inflammatory component (pleuritis or pericarditis). Prednisone (1–2mg/kg/day) or an equivalent drug is used for at least four to six weeks, and subsequently tapered off to its discontinuation.15,18 Doses of prednisone or an equivalent drug should be within the higher recommended ranges due to their interaction with rifampicin.
Respiratory isolation measuresCongenital TB is associated with high bacillary loads in the respiratory secretions and gastric juices of the neonate, and requires isolation measures in the patient's environment, especially during stays in neonatal units, until smears become negative for acid-fast bacilli.19 Thus, it is recommended that a follow-up acid-fast smear is performed 15 days after initiation of antituberculosis treatment, to be repeated at 1 month if the result remains positive. On the other hand, postnatal TB is rarely contagious and does not require isolation measures20 except in patients with positive acid-fast smears from gastric juice or bronchial aspirate samples.
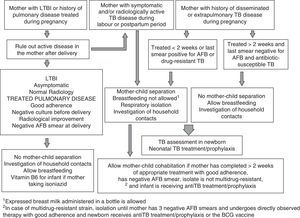
The measures to be taken in newborns or infants exposed to a potentially contagious patient with tuberculosis disease, usually the mother, are summarised in Fig. 1.2,15,18
Vaccination with Bacille Calmette-GuérinThe BCG vaccine is contraindicated during pregnancy.11 The WHO recommends that newborns are routinely vaccinated in highly endemic countries.21 In Spain, this vaccine is not included in the immunisation schedule of any autonomous community, and its administration should only be considered under the following circumstances: (1) travel to countries with a high TB burden and where the BCG vaccine is part of the routine immunisation schedule, for a prolonged stay (>3 months) or with the purpose of settling; (2) exposure to the mother or other household member with multidrug-resistant or extensively drug-resistant TB, given the risk of TB relapse in the household, even when treated adequately17; (3) neonatal LTBI by a multidrug-resistant or extensively drug-resistant strain, as the efficacy of prophylaxis in these cases has not been demonstrated and the vaccine may prevent disseminated forms of TB in the early years of life,17 and (4) prolonged and close contact with a patient diagnosed with infectious TB and poor adherence to treatment, when other preventive measures cannot be applied or have failed. Under these circumstances, if the newborn is receiving primary or secondary prophylaxis, the vaccine will be administered once prophylaxis has been completed.
Monitoring of infants with latent tuberculosis infection or tuberculosis diseaseThe medical follow-up of infants being treated with antituberculosis drugs consists of frequent visits (at least monthly at the beginning of treatment) to monitor patient symptoms, check adherence to treatment and identify potential toxicity. Periodical blood tests are recommended to monitor liver function (isoniazid, rifampicin and pyrazinamide), hyperuricaemia (pyrazinamide) and kidney function (aminoglycosides). In patients receiving aminoglycosides, an auditory evoked potential test should be done at the end of treatment, while it is recommended that patients treated with ethambutol undergo an ophthalmological assessment including a visual evoked potential test.17
If the patient with TB shows adequate clinical and radiological improvement, it is not necessary to systematically repeat the chest X-ray, and clinical follow-up should continue until at least 2 years of age.
Conflicts of interestThe authors have no conflicts of interest to declare.
Working Group on Gestational, Congenital and Postnatal Tuberculosis of the SEIP: Ana Alarcón Allen, Servicio de Neonatología, Hospital Universitari Sant Joan de Déu, Esplugues de Llobregat, Barcelona and Neonatal Unit, Oxford University Hospitals NHS Trust, United Kingdom; Fernando Álvez González, Unidad de Infectología y Vacunas, GENVIP, Hospital Clínico Universitario Santiago de Compostela, A Coruña; Fernando Baquero-Artigao, Servicio de Pediatría, Enfermedades Infecciosas y Patología Tropical, Hospital Infantil La Paz, Madrid; Daniel Blazquez Gamero, Sección de Inmunodeficiencias y Niños Pequeños, Servicio de Pediatría, Hospital 12 de Octubre, Madrid; Marta Cabrera Lafuente, Servicio de Neonatología, Hospital La Paz, Madrid; José Antonio Couceiro Gianzo, Unidad de Infectología, Servicio de Pediatría, Complexo Hospitalario de Pontevedra; María de la Calle Fernández-Miranda, Unidad de Tocología de Alto Riesgo, Servicio de Obstetricia y Ginecología, Hospital La Paz, Madrid; Teresa del Rosal Rabes, Servicio de Pediatría, Enfermedades Infecciosas y Patología Tropical, Hospital Infantil La Paz, Madrid; Claudia Fortuny Guasch, Unidad de Infecciones, Servicio de Pediatría, Hospital Sant Joan de Déu, Universitat de Barcelona, Esplugues de Llobregat, Barcelona; Anna Goncé Mellgren, Servicio de Medicina Maternofetal, Institut Clínic de Ginecologia, Obstetrícia i Neonatologia, Hospital Clínic, Barcelona; Teresa Hernández-Sampelayo Matos, Sección de Enfermedades Infecciosas, Servicio de Pediatría, Hospital Gregorio Marañón, Madrid; Andrea Martín-Nalda, Unidad de Patología Infecciosa e Inmunodeficiencias de Pediatría, Hospital Materno-Infantil Vall d’Hebron, Barcelona; Leticia Martínez Campos, Unidad de Infectologia Pediátrica, Hospital La Inmaculada de Huercal Overa, AGS Norte de Almería; María José Mellado Peña, Servicio de Pediatría, Enfermedades Infecciosas y Patología Tropical, Hospital Infantil La Paz, Madrid; María Mendez Hernández, Servicio de Pediatría, Unidad de Enfermedades Infecciosas e Inmunología Clínica, Hospital Universitario Germans Trias i Pujol, Universidad Autónoma de Barcelona; David Moreno Pérez, Unidad de Infectología, Hospital Materno-Infantil Carlos Haya, Málaga; María Luisa Navarro Gómez, Sección de Enfermedades Infecciosas, Servicio de Pediatría, Hospital Gregorio Marañón, Madrid; Antoni Noguera Julián, Unidad de Infecciones, Servicio de Pediatría, Hospital Sant Joan de Déu, Universitat de Barcelona, Esplugues de Llobregat, Barcelona; Félix Omeñaca Teres, Servicio de Neonatología, Hospital La Paz, Madrid; José Tomás Ramos Amador, Servicio de Pediatría, Hospital Clínico San Carlos, Madrid.
The members of Grupo de trabajo de tuberculosis gestacional, congénita y posnatal de la Sociedad Española de Infectología Pediátrica (SEIP) are presented in Annex 1.
Please cite this article as: Baquero-Artigao F, Mellado Peña MJ, del Rosal Rabes T, Noguera Julián A, Goncé Mellgren A, de la Calle Fernández-Miranda M, et al. Guía de la Sociedad Española de Infectología Pediátrica sobre tuberculosis en la embarazada y el recién nacido (ii): profilaxis y tratamiento. An Pediatr (Barc). 2015;83:286.e1–286.e7.