Although critically ill children may be at risk from developing deep venous thrombosis (DVT), data on its incidence and effectiveness of thromboprophylaxis are lacking.
ObjectiveTo describe the use of thromboprophylaxis in critically ill children in Spain and Portugal, and to compare the results with international data.
Material and methodsSecondary analysis of the multinational study PROTRACT, carried out in 59 PICUs from 7 developed countries (4 from Portugal and 6 in Spain). Data were collected from patients less than 18years old, who did not receive therapeutic thromboprophylaxis.
ResultsA total of 308 patients in Spanish and Portuguese (Iberian) PICUS were compared with 2176 admitted to international PICUs. Risk factors such as femoral vein (P=.01), jugular vein central catheter (P<.001), cancer (P=.03), and sepsis (P<.001) were more frequent in Iberian PICUs. The percentage of patients with pharmacological thromboprophylaxis was similar in both groups (15.3% vs. 12.0%). Low molecular weight heparin was used more frequently in Iberian patients (P<.001). In treated children, prior history of thrombosis (P=.02), femoral vein catheter (P<.001), cancer (P=.02) and cranial trauma or craniectomy (P=.006), were more frequent in Iberian PICUs. Mechanical thromboprophylaxis was used in only 6.8% of candidates in Iberian PICUs, compared with 23.8% in the international PICUs (P<.001).
ConclusionsDespite the presence of risk factors for DVT in many patients, thromboprophylaxis is rarely prescribed, with low molecular weight heparin being the most used drug. Passive thromboprophylaxis use is anecdotal. There should be a consensus on guidelines of thromboprophylaxis in critically ill children.
Los niños críticos pueden tener riesgo de trombosis venosa profunda (TVP), pero no conocemos ni su incidencia ni la eficacia de la tromboprofilaxis.
ObjetivoDescribir la tromboprofilaxis en niños críticos en España y Portugal, en comparación con datos internacionales.
Material y métodosAnálisis secundario del estudio multinacional PROTRACT, realizado en 59 unidades de cuidados intensivos pediátricos (UCIP) de 7 países desarrollados (4 de Portugal y 10 de España). Se incluyeron los pacientes menores de 18años que no recibieran anticoagulación terapéutica.
ResultadosSe analizaron 308 pacientes, que se compararon con 2.176 de UCIP internacionales. Los factores de riesgo: catéter en vena femoral (p=0,01), yugular (p<0,001), cáncer (p=0,03) y sepsis (p<0,001) fueron más frecuentes en las UCIP ibéricas. El porcentaje de pacientes con tromboprofilaxis farmacológica fue similar en ambos grupos (15,3% vs. 12,0%). La heparina de bajo peso molecular se utilizó con mayor frecuencia en las UCIP ibéricas (p<0,001). En los pacientes con profilaxis, la historia de trombosis (p=0,02), catéter venoso femoral (p<0,001), cáncer (p=0,02) y trauma craneal o craneotomía (p=0,006) fueron más frecuentes en las UCIP ibéricas. En solo el 6,8% de los candidatos de las UCIP ibéricas se utilizó tromboprofilaxis mecánica, en comparación con el 23,8% de los internacionales (p<0,001).
ConclusionesA pesar de que los pacientes ingresados en UCIP ibéricas suelen presentar factores de riesgo de TVP, la tromboprofilaxis es poco utilizada, siendo la heparina de bajo peso molecular la medida más habitual. La tromboprofilaxis pasiva se utiliza raramente. Se deberían consensuar las pautas de tromboprofilaxis en los niños críticamente enfermos.
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are severe but often unrecognised complications in critically ill hospitalised patients.1,2 In adult patients admitted to the ICU, the prevalence of DVT or PE with evident clinical manifestations exceeds 20 cases per 1000 patients, with an incidence higher than 14.5 cases per 1000 patients despite the use of pharmacological thromboprophylaxis (PTP),3 so its use is justified unless there is a recognised contraindication for anticoagulation.4
Using the scarce data available for children admitted to paediatric intensive care units (PICUs), the prevalence of DVT or PE with clinical manifestations is estimated at 9 cases per 1000 patients and their incidence at 7 cases per 1000 patients,5 and there are no specific recommendations for the use of PTP in these patients.6
A survey of paediatric intensivists in the United States showed that they were more likely to prescribe PTP to adolescents receiving mechanical ventilation, with a hypercoagulable state, a prior history of DVT or a cavopulmonary anastomosis.7
The PROTRACT prospective multicentre study concluded that the low frequency and great variability in the use of thromboprophylaxis in PICUs call for well-designed research that can set the foundation for establishing the indication of PTP in children.1 In the secondary analysis of the PROTRACT data performed in our study,1 we evaluated the frequency of thromboprophylaxis in critically ill children in Spain and Portugal in an attempt to determine the patient and PICU characteristics associated with its use.
Materials and methodsStudy designWe performed a secondary analysis of the data of the PROTRACT1 prospective observational multicentre multinational study, which was carried out over four study dates in 2012 (February 1st, May 1st, August 1st, and November 1st) in 59 PICUs of 7 developed countries (United States of America, Canada, Australia, New Zealand, Singapore, Portugal and Spain) and included 2484 patients. Our study analysed the data of the patients corresponding to the 4 Portuguese PICUs and the 10 Spanish PICUs included in the PROTRACT study, and compared it to the data of the remaining patients (those hospitalised in PICUs of the remaining countries).
The methodology of the PROTRACT study has been published in full.1 The study included all patients less than 18 years old hospitalised in the PICU during the study dates, unless they were receiving therapeutic anticoagulation or boarding in the unit for lack of available beds elsewhere in the hospital. The data for each patient were collected at 9am on study dates, and included data on demographic characteristics, preadmission history, and the clinical features of the current admission. The study collected data on clinical features with a known association with DVT or PE,2,6,8–11 indications and contraindications for PTP based on the paediatric recommendations of the American College of Chest Physicians (ACCP),6 and the actual prescription of thromboprophylaxis. It also counted the number of central venous catheters (CVCs) used in patients, as their presence is the most significant risk factor for DVT in children.12,13 The study documented any current treatments that may have been associated with a risk of DVT and may have affected the prescription or the outcome of thromboprophylaxis (vasoactive agents, parenteral nutrition, mechanical ventilation, l-asparaginase therapy, bed rest, and previous or scheduled surgery). The data were collected and handled using the Research Electronic Data Capture (REDCap) system of Washington University and the St. Louis School of Medicine.14
Definitions and outcome measuresWe defined a patient as a candidate for PTP if: (a) the patient was hospitalised in the PICU and (b) based on the paediatric recommendations of the ACCP, he or she had a dilated cardiomyopathy, cavopulmonary anastomosis, cyanotic congenital heart disease, end-stage renal disease or pulmonary hypertension, with no contraindications for anticoagulation. Anticoagulation was considered contraindicated in case of: scheduled surgery, heparin-induced thrombocytopaenia, or intracranial or extracranial haemorrhage requiring blood transfusions.6,15
We considered that any patient that received a thromboprophylactic agent (aspirin, low molecular weight heparin, intravenous or subcutaneous unfractionated heparin, warfarin or clopidogrel) or mechanical thromboprophylaxis in the 24h prior to the study date was receiving thromboprophylaxis. The administration of more than 10 units per hour of unfractionated heparin through the CVC was considered a thromboprophylactic dose, while lower doses were considered a treatment for maintaining CVC patency.12
The primary outcome measure was the prevalence of PTP, defined as the proportion of all patients hospitalised in the PICU that were receiving PTP. Our secondary objective was to identify the factors associated with the prescription of thromboprophylaxis. The analysis of mechanical thromboprophylaxis only included children older than 8 years, the age at which this therapy starts to be applied.12
Statistical analysisPatients were divided into 3 age categories: infants younger than one year, children aged 1–13 years, and adolescents older than 13 years, based on the bimodal distribution of DVT in children.12,13 We pooled the data for the 4 study dates. Continuous variables are expressed as median and interquartile range (IQR), while categorical variables are expressed as absolute frequencies and percentages. The statistical analysis was performed with the Stata 12 software (StataCorp LP, College Station, TX). Statistical significance was defined as P<.05 for two-tailed tests. We calculated the odds ratios and the 95% confidence intervals. The full details of the statistical analysis have been published previously.1
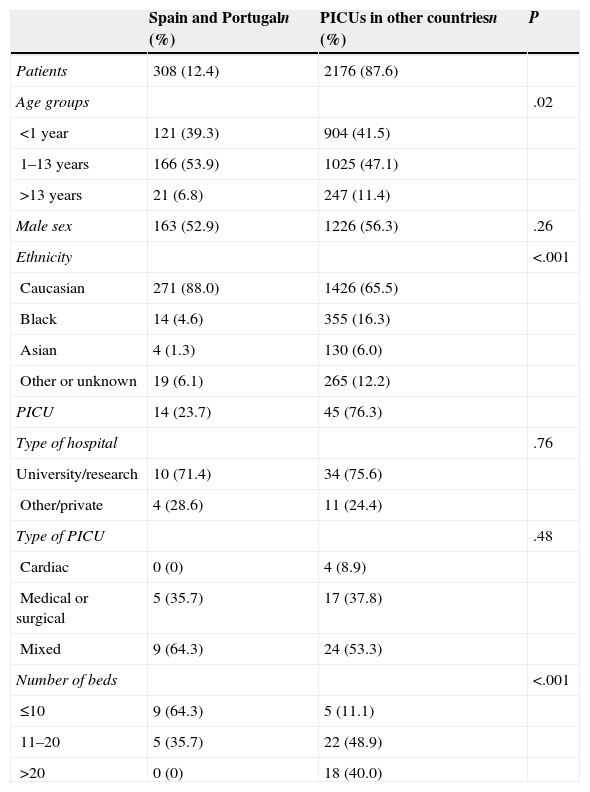
ResultsCharacteristics of the patients and the paediatric intensive care unitsThe study included a total of 308 (12.4%) patients admitted to 14 PICUs in Spain and Portugal (Iberian PICUs) that were compared to 2176 (87.6%) patients admitted to 45 PICUs in other countries (international PICUs). The patients had a similar distribution across the 4 study dates. Table 1 summarises the patient and PICU characteristics. The distribution in age groups was significantly different in the two subgroups, with a greater percentage of patients aged less than 1 year and more than 13 years in the international PICUs (P=.02). As for ethnicity, while only 6.2% of patients in the Iberian PICUs were non-white, 24.3% of them were non-white in international PICUs (P<.001). The size of the PICUs was very different, since most PICUs in Portugal and Spain (64.3%) had 10 or fewer beds, while 88.9% of international PICUs had more than 10 beds (P<.001).
Characteristics of the patients and paediatric intensive care units (PICUs). Results given as absolute frequencies and percentages.
| Spain and Portugaln (%) | PICUs in other countriesn (%) | P | |
|---|---|---|---|
| Patients | 308 (12.4) | 2176 (87.6) | |
| Age groups | .02 | ||
| <1 year | 121 (39.3) | 904 (41.5) | |
| 1–13 years | 166 (53.9) | 1025 (47.1) | |
| >13 years | 21 (6.8) | 247 (11.4) | |
| Male sex | 163 (52.9) | 1226 (56.3) | .26 |
| Ethnicity | <.001 | ||
| Caucasian | 271 (88.0) | 1426 (65.5) | |
| Black | 14 (4.6) | 355 (16.3) | |
| Asian | 4 (1.3) | 130 (6.0) | |
| Other or unknown | 19 (6.1) | 265 (12.2) | |
| PICU | 14 (23.7) | 45 (76.3) | |
| Type of hospital | .76 | ||
| University/research | 10 (71.4) | 34 (75.6) | |
| Other/private | 4 (28.6) | 11 (24.4) | |
| Type of PICU | .48 | ||
| Cardiac | 0 (0) | 4 (8.9) | |
| Medical or surgical | 5 (35.7) | 17 (37.8) | |
| Mixed | 9 (64.3) | 24 (53.3) | |
| Number of beds | <.001 | ||
| ≤10 | 9 (64.3) | 5 (11.1) | |
| 11–20 | 5 (35.7) | 22 (48.9) | |
| >20 | 0 (0) | 18 (40.0) |
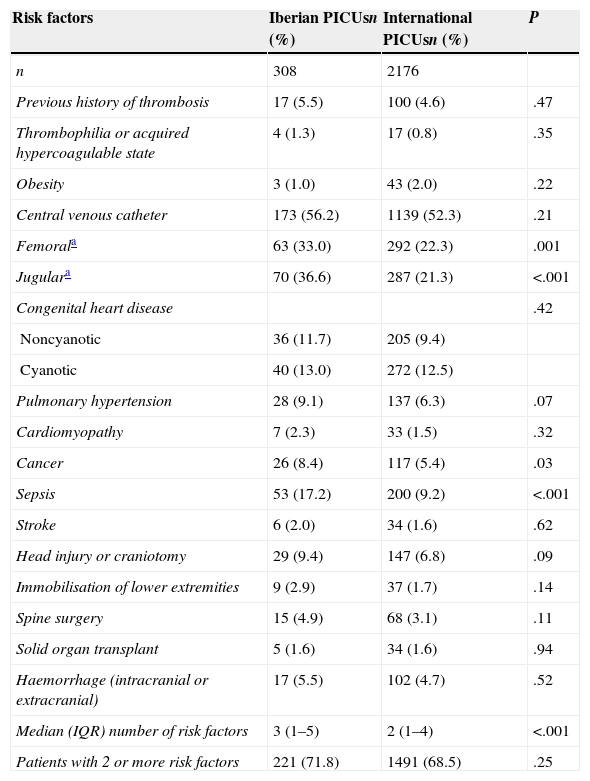
The risk factors for DVT found in patients are shown in Table 2. When we compared the two samples, we found that femoral (P=.01) or jugular vein catheters (P<.001), cancer (P=.03) and sepsis (P<.001) were found more frequently in Spanish and Portuguese PICUs. The median number of risk factors was significantly higher in these PICUs than in international PICUs (3 vs. 2; P<.001), although the percentages of patients with more than 2 coexisting risk factors were similar (Table 2).
Risk factors for deep vein thrombosis. Results in absolute frequencies and percentages.
| Risk factors | Iberian PICUsn (%) | International PICUsn (%) | P |
|---|---|---|---|
| n | 308 | 2176 | |
| Previous history of thrombosis | 17 (5.5) | 100 (4.6) | .47 |
| Thrombophilia or acquired hypercoagulable state | 4 (1.3) | 17 (0.8) | .35 |
| Obesity | 3 (1.0) | 43 (2.0) | .22 |
| Central venous catheter | 173 (56.2) | 1139 (52.3) | .21 |
| Femorala | 63 (33.0) | 292 (22.3) | .001 |
| Jugulara | 70 (36.6) | 287 (21.3) | <.001 |
| Congenital heart disease | .42 | ||
| Noncyanotic | 36 (11.7) | 205 (9.4) | |
| Cyanotic | 40 (13.0) | 272 (12.5) | |
| Pulmonary hypertension | 28 (9.1) | 137 (6.3) | .07 |
| Cardiomyopathy | 7 (2.3) | 33 (1.5) | .32 |
| Cancer | 26 (8.4) | 117 (5.4) | .03 |
| Sepsis | 53 (17.2) | 200 (9.2) | <.001 |
| Stroke | 6 (2.0) | 34 (1.6) | .62 |
| Head injury or craniotomy | 29 (9.4) | 147 (6.8) | .09 |
| Immobilisation of lower extremities | 9 (2.9) | 37 (1.7) | .14 |
| Spine surgery | 15 (4.9) | 68 (3.1) | .11 |
| Solid organ transplant | 5 (1.6) | 34 (1.6) | .94 |
| Haemorrhage (intracranial or extracranial) | 17 (5.5) | 102 (4.7) | .52 |
| Median (IQR) number of risk factors | 3 (1–5) | 2 (1–4) | <.001 |
| Patients with 2 or more risk factors | 221 (71.8) | 1491 (68.5) | .25 |
IQR: interquartile range.
Out of the 14 analysed PICUs, 9 (64.3%) had a formal thromboprophylaxis protocol, compared to 26 out of the 45 (57.8%) international PICUs (P=.67).
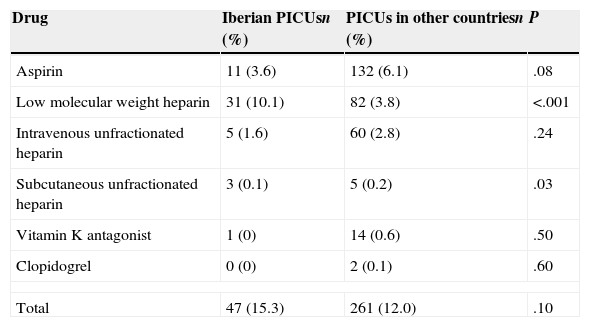
Pharmacological thromboprophylaxisA total of 47 patients (15.3%) in Iberian PICUs received some type of PTP, compared to 261 (12.0%) in the cohort of the other countries (P=.10). The drugs used are shown in Table 3. Low molecular weight heparin was used most frequently in patients treated in Iberian PICUs than in PICUs in other countries (P<.001), while subcutaneous unfractionated heparin was used more frequently in the latter, although the number of cases in which it was used was very small, so the data cannot be used to draw any valuable conclusions.
Pharmacological thromboprophylaxis measures used.
| Drug | Iberian PICUsn (%) | PICUs in other countriesn (%) | P |
|---|---|---|---|
| Aspirin | 11 (3.6) | 132 (6.1) | .08 |
| Low molecular weight heparin | 31 (10.1) | 82 (3.8) | <.001 |
| Intravenous unfractionated heparin | 5 (1.6) | 60 (2.8) | .24 |
| Subcutaneous unfractionated heparin | 3 (0.1) | 5 (0.2) | .03 |
| Vitamin K antagonist | 1 (0) | 14 (0.6) | .50 |
| Clopidogrel | 0 (0) | 2 (0.1) | .60 |
| Total | 47 (15.3) | 261 (12.0) | .10 |
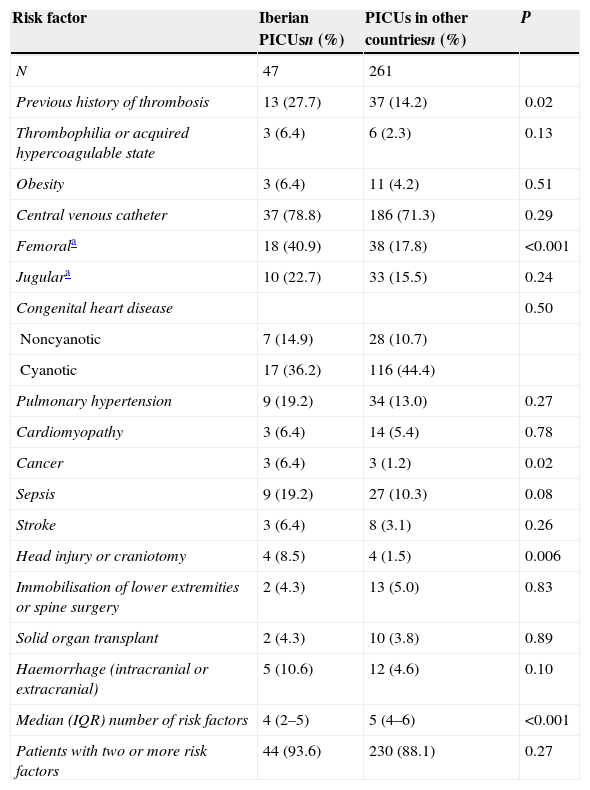
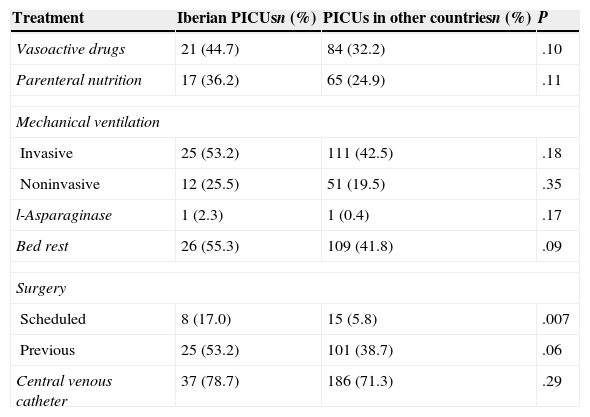
Table 4 shows the risk factors of the patients, and Table 5 the treatments patients were receiving. In Iberian PICUs, the most frequent factors were a previous history of thrombosis (P=.02), the presence of a femoral venous central catheter (P<.001), cancer (P=.02) and head injury or craniotomy (P=.006), while the median number of risk factors in patients that received PTP was higher in international PICUs (5 vs. 4; P<.001). The presence of concomitant treatments was similar in both series, with the exception of scheduled surgery (patients admitted to the PICU with a surgery scheduled within the next two days), which was more frequent in Iberian PICUs (P=.007).
Risk factors for deep vein thrombosis in patients that received pharmacological thromboprophylaxis.
| Risk factor | Iberian PICUsn (%) | PICUs in other countriesn (%) | P |
|---|---|---|---|
| N | 47 | 261 | |
| Previous history of thrombosis | 13 (27.7) | 37 (14.2) | 0.02 |
| Thrombophilia or acquired hypercoagulable state | 3 (6.4) | 6 (2.3) | 0.13 |
| Obesity | 3 (6.4) | 11 (4.2) | 0.51 |
| Central venous catheter | 37 (78.8) | 186 (71.3) | 0.29 |
| Femorala | 18 (40.9) | 38 (17.8) | <0.001 |
| Jugulara | 10 (22.7) | 33 (15.5) | 0.24 |
| Congenital heart disease | 0.50 | ||
| Noncyanotic | 7 (14.9) | 28 (10.7) | |
| Cyanotic | 17 (36.2) | 116 (44.4) | |
| Pulmonary hypertension | 9 (19.2) | 34 (13.0) | 0.27 |
| Cardiomyopathy | 3 (6.4) | 14 (5.4) | 0.78 |
| Cancer | 3 (6.4) | 3 (1.2) | 0.02 |
| Sepsis | 9 (19.2) | 27 (10.3) | 0.08 |
| Stroke | 3 (6.4) | 8 (3.1) | 0.26 |
| Head injury or craniotomy | 4 (8.5) | 4 (1.5) | 0.006 |
| Immobilisation of lower extremities or spine surgery | 2 (4.3) | 13 (5.0) | 0.83 |
| Solid organ transplant | 2 (4.3) | 10 (3.8) | 0.89 |
| Haemorrhage (intracranial or extracranial) | 5 (10.6) | 12 (4.6) | 0.10 |
| Median (IQR) number of risk factors | 4 (2–5) | 5 (4–6) | <0.001 |
| Patients with two or more risk factors | 44 (93.6) | 230 (88.1) | 0.27 |
IQR: interquartile range.
Concomitant treatments in patients receiving pharmacological thromboprophylaxis.
| Treatment | Iberian PICUsn (%) | PICUs in other countriesn (%) | P |
|---|---|---|---|
| Vasoactive drugs | 21 (44.7) | 84 (32.2) | .10 |
| Parenteral nutrition | 17 (36.2) | 65 (24.9) | .11 |
| Mechanical ventilation | |||
| Invasive | 25 (53.2) | 111 (42.5) | .18 |
| Noninvasive | 12 (25.5) | 51 (19.5) | .35 |
| l-Asparaginase | 1 (2.3) | 1 (0.4) | .17 |
| Bed rest | 26 (55.3) | 109 (41.8) | .09 |
| Surgery | |||
| Scheduled | 8 (17.0) | 15 (5.8) | .007 |
| Previous | 25 (53.2) | 101 (38.7) | .06 |
| Central venous catheter | 37 (78.7) | 186 (71.3) | .29 |
Mechanical thromboprophylaxis was performed in only 5 out of 73 (6.8%) candidate patients (aged more than 8years) in Iberian PICUs, compared to 156 out of 655 (23.8%) in international PICUs (P<.001). Compression stockings were used in all instances in Spain and Portugal, while sequential pneumatic compression devices were used in 85.9% and compression stockings in 22.4% of the patients in other countries (P<.001).
DiscussionVenous thromboembolism is a significant cause of morbidity and mortality in hospitalised adults, with reported incidence rates ranging from 10% to 40%.2,16 The incidence in children seems to be much lower at about 0.5% of the total number of hospitalisations.2,17–19 When children are critically ill, the risk factors for DVT increase in number, so it is likely that its incidence also increases, although no reliable data are available on this particular.1
In light of this, the use of a thromboprophylaxis regimen in children admitted to the PICU may be justified, although this remains a matter of contention. On one hand, its indiscriminate use could unnecessarily expose low-risk children to an increased risk of bleeding or complications such as heparin-induced thrombocytopaenia.2 On the other hand, failure to use of thromboprophylaxis in high-risk children could result in episodes of DVT or PE that could have been prevented.2 In addition to pharmacological measures, mechanical thromboprophylaxis (which is usually indicated starting at 8 years of age) is a preventive measure available to the patient that poses no risks.
Intensivists acknowledged that the criterion they use for prescribing thromboprophylaxis in actual practise is the presence of one or more risk factors, such as the patient having a CVC, mechanical ventilation, heart disease, or hypergoagulability.1,7,20
The PROTRACT multicentre study, which included a considerable number of PICUs and patients from various developed countries and of which our study made a secondary analysis, demonstrated the great variability that exists in the use of thromboprophylaxis in critically ill children.1 It identified a series of characteristics associated to the use of PTP, such as congenital heart disease, immobility, obesity, a previous history of thrombosis or cavopulmonary anastomosis, the presence of one or more CVCs, and adolescence.1
However, given the aforementioned variability, we had to compare the results obtained in the PICUs in our region (the Iberian Peninsula) to gain a more accurate perspective on what occurs in our units. Our results show that the characteristics of Iberian PICUs and their patients differ significantly from the rest of the sample, with Iberian PICUs having fewer beds, a lower percentage of patients in the extreme age groups (younger than 1 year and older than 13 years) and a small percentage of non-white patients. Still, the risk factors for DVT found in patients in the Iberian Peninsula were similar to those found in patients of PICUs in other countries, although we found a significantly higher frequency for factors such as CVCs (femoral and jugular), cancer and sepsis. While our study was not designed to establish or rule out causality, we believe that the number of beds in the PICUs or the age of the patients should probably not be considered relevant factors.
While the percentage of patients who received PTP was similar in Iberian and international PICUs, the regimens applied diverged, with low molecular weight heparin being the most frequently used drug in Iberian PICUs and aspirin in international PICUs, which shows greater compliance of Iberian PICUs with current recommendations.1,6
Many of the patients admitted to the PICU have complex clinical conditions, and may have more than one risk factor for DVT. In our series, the median number of risk factors was 3, while in the multinational PICU study the median was 2. The median number in patients that did receive PTP was higher in countries other than Spain and Portugal (5 vs. 4).
Despite their simplicity (as in using graduated compression stockings) and their safety (since they do not carry a risk of bleeding), mechanical thromboprophylaxis measures were used rarely in Iberian PICUs, while they were used in one-fourth of candidate patients in the international PICUs. Although there is no conclusive evidence on their efficacy,1 we believe that they offer an opportunity to improve the prevention of DVT that should be taken into consideration by intensivists in children aged more than 8 years that have any risk factors.
Why is thromboprophylaxis used so rarely in critically ill children? Several factors are probably at play, such as the lack of evidence for the paediatric age group, underestimation of the risk of DVT by paediatricians, current protocols being based on expert opinions (and therefore less binding) and the varying compliance with some recommendations found in clinical practise,1,6 as well as the concern of bleeding following administration of heparin or other antiaggregant or anticoagulant drugs.7,17 Having a formal protocol for thromboprophylaxis would contribute to the homogeneous implementation of these measures. Such protocols, which are in place in over half of the PICUs in Portugal, Spain, and the remaining countries, have the drawback of being necessarily based on the extrapolation of adult data and protocols and the opinions and personal preferences of the professionals in each unit.1
There are limitations to our results that need to be taken into account. As happened in the multinational PROTRACT study, we did not gather data on the clinical outcome of patients as it relates to DVT, PE or bleeding, as the study was designed solely to determine the presence of risk factors and the thromboprophylactic measures applied. Thus, it would be interesting to conduct another study with a large enough sample of PICUs with a methodology that would make it possible to establish the clinical significance of the risk factors for DVT and PE as well as the risks and benefits (individual and comparative) of different thromboprophylactic measures.1 Such a study could provide the foundation to develop an evidence-based thromboprophylaxis protocol for the PICU.
Participation of the PICUs was voluntary, which may have led to a bias in patient characteristics. In any case, based on the characteristics of the participating Portuguese and Spanish PICUs we believe that they provide an accurate reflection of intensive care in their countries. As for the potential heterogeneity of PICUs in other countries, we consider that while their structure and work may be organised differently, since they all are in developed countries they may be comparable in terms of human and material resources, the type of admitted patients, and the procedures performed. The fact that due to administrative reasons the Portuguese and Spanish PICUs are smaller (have fewer beds) than those of other countries may have biased the results slightly, so this should be taken into account.
ConclusionsAlthough many critically ill children have more than one risk factor for DVT, thromboprophylactic measures are used infrequently in this set of patients, both in Spain and Portugal and at the international level. The use of low molecular weight heparin in Spanish and Portuguese PICUs is more frequent and widespread and mechanical thromboprophylaxis is rarely used, while the latter is used more frequently in PICUs from other countries. An effort should be made to reach a consensus on the indications and regimens of thromboprophylaxis in critically ill children.
FundingDr. E.V. Faustino has received support from the National Center for Advancing Translational Sciences under award numbers UL1TR000142 and KL2TR000140.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank every participant in the PROTRACT study for their collaboration in our study.
Akron Children's Hospital: Ann-Marie Brown, MSN; Alberta Children's Hospital: Leena Desai, MSc, and Elaine Gilfoyle, MD, MMEd; Baylor College of Medicine: Michelle Goldsworthy, BN, Nancy Jaimon, BSN, MSN, and Laura Loftis, MD; Baystate Medical Center: Michael Canarie, MD; Boston Children's Hospital: Daniel Kelly, MD, and Adrienne Randolph, MD, MSc; Centre Hospitalier Universitaire de Sherbrooke: Miriam Santschi, MD, MSc; Centre Mere-Enfant Soleil du CHU de Quebec: Marc-Andre Dugas, MD, MSc, and Louise Gosselin, BN; Centro Hospitalar Lisboa Norte: Joana Rios, MD; Children's Hospital and Medical Center, Omaha and Nebraska Medical Center: Edward Truemper, MD, MS, and Brenda Weidner, BSN; Children's Hospital and Research Center Oakland: Heidi Flori, MD, and Julie Simon, RN; Children's Hospital at Westmead: Marino Festa, MD, Karen Walker, PhD, and Nicola Watts, PhD; Children's Hospital of Philadelphia: Daniela Davis, MD, MSCE, Mary Ann DiLiberto, BS, William Kamens, BA, Rebecca McIntosh, BA, Brooke Park, BSN, and Janice Prodell, RN; Children's Hospital of Wisconsin: Sheila Hanson, MD, MS, Kathleen Murkowski, BS, and David Triscari, BS; Children's Hospitals and Clinics of Minnesota: Chris Leonard, BA, Jeffrey Nowak, MD, and Alison Overman, BA; CHU Sainte-Justine University of Montreal: Mariana Dumitrascu, MD, Jacques Lacroix, MD, and Marisa Tucci, MD; Cohen Children's Medical Center of New York: Aaron Kessel, MD, James Schneider, MD, and Todd Sweberg, MD; Complexo Hospitalario Universitario A Coruna: Angela Ferrer Barba, MD, and Carmen Ramil, MD; Connecticut Children's Medical Center: Christopher Carroll, MD, MS; Dell Children's Medical Center of Central Texas: LeeAnn Christie, MSN, and Renee Higgerson, MD; Doernbecher Children's Hospital: Aileen Kirby, MD; Duke University Medical Center: Ira Cheifetz, MD, Kyle Rehder, MD, and Samantha Wrenn, BS; Gregorio Maranon General University Hospital: Jesus Lopez-Herce, MD, PhD; Helen DeVos Children's Hospital: Nabil Hassan, MD, and Akunne Ndika, MBBS, MPH; Hospital Clínico Universitario de Santiago: Antonio Rodriguez-Nunez, MD, PhD, and Sara Trabazo-Rodriguez, MD; Hospital Dona Estefania: Maria Ventura, MD; Hospital Infantil Universitario Miguel Servet: Juan Pablo Garcia Íñiguez, MD, and Paula Madurga Revilla, MD; Hospital Infantil Universitario Niño Jesús: Maria Isabel Iglesias Bouzas, MD; Hospital Pediatrico Coimbra: Maria Dionisio, MD; Hospital Regional Universitario Materno Infantil Carlos Haya: Patricia García Soler, MD; Hospital Sao Joao: Miguel Fonte, MD; Hospital Universitario Materno Infantil Las Palmas de Gran Canaria: Antonio Jimenez, MD; Joseph M Sanzani Children's Hospital at Hackensack University Medical Center: Shira Gertz, MD; KK Women's and Children's Hospital: Loh Tsee Foong, MBBS, MMed, and Anuradha Menon, MBBS; Maria Fareri Children's Hospital: Simon Li, MD, MPH; Mater Children's Hospital: Sara Mayfield, BHSc, and Andreas Schibler, MD; Montreal Children's Hospital: Yasser Kazzaz, MBBS, and Samara Zavalkoff, MDCM; Nationwide Children's Hospital: Sue Cunningham, BSN, Kristin Greathouse, MS, BSN, Dianna Hidalgo, BS, Sarah O’Brien, MD, MSc, Kami Perdue, BS, and Lisa Steele, BSN; Nuestra Señora de Candelaria Hospital: Jose Sebastian Leon Gonzalez, MD; Penn State Children's Hospital: Debra Spear, RN, and Robert Tamburro, MD; Princess Margaret Hospital: Simon Erickson, MBBS; Riley Hospital for Children: Stephanie Fritz, RN, Christi Rider, LPN, and Mark Rigby, MD, PhD; Royal Children's Hospital Brisbane: Debbie Long, PhD, Anthony Slater, MBBS, and Tara Williams, BSN; Royal Children's Hospital Melbourne: Warwick Butt, MBBS, Carmel Delzoppo, BSc, and Sophie Syddall, PhD; Sant Joan de Deu Hospital: Iolanda Jordan, PhD, and Lluisa Hernandez, MD; St. Louis Children's Hospital: Rachel Jacobs, BA, Philip C. Spinella, MD, and Cindy Terrill, CCRP; Starship Children's Hospital: John Beca, MBChB, Tracey Bushell, BA, Miriam Rea, BN, and Claire Sherring, RN; Stony Brook University Medical Center: Kathleen Culver, DNP, and Margaret Parker, MD; Sydney Children's Hospital: Gary Williams, MBBS, and Janelle Young, MApSc, MPH; University Hospital of Salamanca: Mirella Gaboli, MD, PhD, and Pedro Gomez de Quero, MD; University of California at San Francisco: Victoria Lo, BS, and Anil Sapru, MD, MAS; University of Rochester Medical Center: Jill M. Cholette, MD, and L. Eugene Daugherty, MD; University of Virginia: Robin Kelly, RN, and Douglas Willson, MD; Women's and Children's Hospital, Adelaide: Georgia Letton, BA, and Michael Yung, MD; Yale-New Haven Children's Hospital: Edward Vincent S. Faustino, MD, Kim Marohn, MD, Veronika Northrup, MPH, Li Qin, PhD, Sree Pemira, MD, Joana Tala, MD, Elyor Vidal, MPH.
The researchers of the PROTRACT international multicentre study are listed in Annex 1.
Please cite this article as: Rodríguez Núñez A, Fonte M, Faustino EVS, en representación de los investigadores del estudio multicéntrico internacional PROTRACT. Utilización de medidas de tromboprofilaxis en niños críticamente enfermos en España y Portugal. An Pediatr (Barc). 2015;82:144–151.