To evaluate cold and cough medications and their suitability in children in Primary Health Care in Area V of the Asturian Health Service.
Material and methodsA cross-sectional, descriptive and retrospective study was conducted in which an analysis was performed of the respiratory diseases and the prescriptions of 6 Primary Health Care paediatricians who worked in Area V of the Asturian Health Service in 2011. An evaluation was made on the suitability of these medications. An analysis was also made of the drug datasheet and clinical recommendations (clinical guidelines, protocols or reports).
ResultsA total of 424 cold and cough drugs: 249 antitussives, 155 mucolytics, and 20 “others” were analysed. The mean age was 5 years old. There was a total of 85.1% unsuitable prescriptions. Off-label drugs were used in 11.6%. The prescribing was considered unsuitable in 82.8% of prescriptions associated with R74 and 73% of R05. All of the prescription drugs in children under 6 years old were unsuitable. Mucolytics/“others” were not suitable in 99.4%, nor antitussives in 75.1%.
ConclusionsThere is a high level of cold and cough drugs being prescribed in children, with 85% of these being unsuitable. Children should only receive drugs with a good risk and benefit ratio. Paediatricians should try to improve the information about paediatric drug use and spread this information to parents, doctors and nurses.
Conocer el perfil de prescripción de anticatarrales en las consultas de Pediatría de Atención Primaria en el Área V del Servicio de Salud del Principado de Asturias y valorar su idoneidad.
Material y métodosEstudio transversal, descriptivo y retrospectivo. Se analizaron las prescripciones para el tratamiento de los procesos respiratorios en 6 consultas de Pediatría de Atención Primaria en el Área Sanitaria V del Servicio de Salud del Principado de Asturias en el año 2011. Se valoraron la idoneidad de los tratamientos prescritos mediante las fichas técnicas de los fármacos y las indicaciones clínicas según el diagnóstico, siguiendo las recomendaciones de guías clínicas, protocolos o en su defecto la bibliografía disponible más actual.
ResultadosSe analizaron 424 anticatarrales: 249 antitusígenos, 155 mucolíticos y 20 clasificados en otros. La media de edad de los pacientes es de 5 años. Un 85,1% de las prescripciones se consideran inadecuadas. El 11,6% de ellos fueron prescritos fuera de ficha técnica. Se consideraron inadecuados el 82,8% de los asociados al diagnóstico R74 y el 73% al R05. Todos los fármacos de los menores de 6 años se consideraron inadecuados. El 99,4% de los mucolíticos/otros y el 75,1% de los antitusígenos se consideraron inadecuados.
ConclusionesSe observa un alto porcentaje de prescripción de fármacos anticatarrales en menores de 14 años en nuestro medio, encontrándose un 85% de las prescripciones inadecuadas. Los niños deberían recibir solo medicamentos con una relación beneficio-riesgo favorable; para ello es necesario mejorar la información sobre el uso pediátrico y promover acciones formativas dirigidas a los padres y a los profesionales sanitarios.
During the last few years, there has been some controversy over the employment of anticatarrhal drugs, due to the use and non-prescription sale of many pharmaceutical drugs with uncertain therapeutic efficacy and a potential risk for intoxication in paediatric-aged patients.1,2
In the year 2008, the United States Food and Drug Administration issued a recommendation to avoid the administration of any drug to combat cough or catarrh in children under the age of two, due to the risk of side effects; their determination on the use of anticatarrhal drugs in children between the ages of two and eleven is still pending.3,4 In other countries, such as Canada, the United Kingdom, Australia, Belgium and The Netherlands, their use has also not been recommended in children under the age of six, and the sale of these non-prescription drugs5 has been prohibited.
The last National Health Survey in Spain, for the period 2011–2012, observed that 48.2% of the population between ages of 0 and 15 had in the previous two weeks consumed drugs to relieve symptoms associated with severe respiratory infection (excluding analgesics-antipyretics and antibiotics); these had not been prescribed by a doctor in more than one quarter of the cases.6
To this, we must add that the accidental ingestion of anticatarrhal drugs is still a frequent reason for consultation due to intoxication in Paediatric Emergencies, and that the use of drugs under different conditions than those authorised still occurs among paediatric patients. In Spain the Ministry of Health qualifies as incorrect use the employment of these drugs under unauthorised conditions and warns about the legal risks that this practice involves.7,8
Based on these premises, we have considered it advisable to perform a study on the prescription profile of anticatarrhal drugs in Primary Care Paediatric consultations (PC) by collecting data from clinical records elaborated by the doctors who assist the paediatric population in our region.
Materials and methodsCharacteristics of the studyThis was a cross-sectional, descriptive, retrospective study, where the respiratory problems and their prescriptions were analysed by reviewing the clinical records using OMI-AP (programme for electronic clinical records used in our autonomous community at health centres) in six PC paediatric offices from the V Healthcare Area belonging to the Sanitary Service of the Principado de Asturias between 1 January and 31 December, 2011.
This area covers the councils of Gijón, Carreño and Villaviciosa. The population is mainly urban, and a total of 31,111 children under the age of 14 were assisted in the year 2011. It comprises 14 basic health areas, with a total of 32 paediatricians. Six offices with access availability during the afternoon, three days a month from Mondays to Fridays were randomly chosen (16 Mondays, 5 Tuesdays, 5 Wednesdays, 4 Thursdays and 6 Fridays).
Patients with respiratory problems were evaluated and the following variables were gathered: (1) patient data: date of birth, age, gender, drug allergies, chronic diseases and/or chronic treatments; (2) reason for the consultation and number of consultations attended for the same problem; (3) diagnosis: CIAP-1 code chosen by the doctor (Table 1), and (4) prescription data: if they were receiving medication at the time of the consultation, the doctor who prescribed the medication and assisted the patient, the active substance and/or commercial name of the drug prescribed in the visit, its presentation and posology.
Frequency of the CIAP-1 codes associated to the use of anticatarrhal drugs.
| ICPC | Description of the diagnosis code | Absolute frequency | Relative frequency |
|---|---|---|---|
| R05 | Cough, expectoration, fever | 37 | 8.7% |
| R23 | Aphonia, dysphonia, voice problems | 1 | 0.2% |
| R72 | Streptococcal PT, scarlet fever | 1 | 0.2% |
| R74 | Catarrh, flu, coryza, pharyngitis, URTI, severe rhinitis, severe rhinopharyngitis | 310 | 72.8% |
| R75 | Sinus infection, sinusitis | 2 | 0.5% |
| R76 | Peritonsillar abscess, severe tonsillitis, PT, herpangina | 3 | 0.7% |
| R77 | Croup, epiglottitis, severe laryngitis, severe tracheitis | 14 | 3.3% |
| R78 | Bronchiolitis, bronchitis, tracheobronchitis | 5 | 1.2% |
| R80 | Flu | 18 | 4.2% |
| R96 | Asthma, allergic/asthmatic bronchitis, hay fever, exercise-induced asthma, asthmatic crisis, allergic rhinitis | 16 | 3.8% |
| R97 | Allergic rhinitis, vasomotor, chronic, pollinosis | 1 | 0.2% |
| H01 | Pain in mastoid area/ear, inner ear pain, outer year pain, ear inflammation, otalgia, ear lobe pain | 1 | 0.2% |
| H71 | Myringitis, suppurative AOM, non-specific AOM, perforated AOM, AOM | 12 | 2.8% |
| H72 | Non-serous otitis media, SOM | 3 | 0.7% |
| H73 | Eustachian tube obstruction/blockage, Eustachian tube infection, tubaritis, ototubaritis Ag/Cr | 1 | 0.2% |
| D83 | Cold sore, aphthous ulcer, mouth disease, tongue, lips, salivary glands, severe stomatitis, glossitis | 1 | 0.2% |
ICPC: International Classification of Primary Care; PT: severe pharyngotonsillitis; URTI: upper respiratory tract infection; AOM: acute otitis media; SOM: serous otitis media.
The suitability of prescribed treatments was assessed taking into consideration two aspects:
- 1.
The data that appeared in the data sheet available on the webpage of the Spanish Agency of Medicines and Medical Devices, or otherwise in the directions available in the 2012 formulary, of all those drugs used for respiratory system problems, including the following subgroups from the ATC classification: R01B corresponding to nasal decongestants of systemic use, and R05, which includes preparations for coughs and flu.
- 2.
At the same time, the suitability of the prescribed treatments for the patients under study was assessed, following the recommendations of clinical guidelines, protocols, or otherwise the most updated bibliography available according to the clinical condition they presented with, sorting them in this manner according to whether it was considered unsuitable or suitable. The use of the drugs under analysis is not recommended in patients with asthma, severe bronchiolitis, severe bronchitis, flu, severe laryngitis, pneumonia, serous otitis media, severe sinusitis and whooping cough.9–24 In cases where there are catarrh symptoms, the use of cough suppressants is not recommended in children under the age of six, although it could be an alternative under medical supervision in some patients over the age of six.25
The statistical analysis was conducted using the computer program SPSS version 15.0, with licence. Absolute and relative frequencies were calculated for qualitative variables, and means, medians and 95% confidence intervals of means were calculated for quantitative variables. For the comparisons between groups, we used the Chi-square test and the binary logistic regression analysis (qualitative variables). A (p) value under 0.05 was considered a statistically significant difference.
ResultsGeneral data of the sampleA total of 1889 patients was analysed in 2659 consultations for respiratory problems, with 2583 pharmacological prescriptions. In 726 consultations no prescriptions were made. The most frequent diagnosis was for upper respiratory tract infection/R74 (42.5% of the consultations), followed by asthma/R96 (16.9%).
Analysis of the anticatarrhal drugs prescribedIn this article, 424 anticatarrhal drugs were analysed (16.4% of all registered prescriptions): 249 cough suppressants (9.6%), 155 mucolytics (6%) and 20 classified under others (0.7%) (Table 2), prescribed for a total of 354 patients. In 23.7% of the consultations, the patient was already taking some previously prescribed medication for the same problem. Of these drugs, 14 cough suppressants, 4 mucolytics and 5 from the others group had been prescribed in the previous visit and were not suspended.
Distribution of prescribed active substances (therapeutic subgroups R01B and R05).
| Drugs (active substance) | Total number | Age groups | |||||
|---|---|---|---|---|---|---|---|
| <6 months | 6 months-1 year | 1–2 years | 2–6 years | 6–12 years | >12 years | ||
| Mucolytics | 155 | ||||||
| Acetylcysteine | 52 | – | – | 4 | 32 | 10 | 6 |
| Ambroxol | 83 | 4 | 12 | 45 | 20 | 2 | |
| Carbocysteine | 20 | 2 | 9 | 6 | 3 | ||
| Cough suppressants | 249 | ||||||
| Cloperastine | 151 | – | – | 6 | 97 | 44 | 4 |
| Dextromethorphan | 47 | 3 | 5 | 18 | 12 | 8 | 1 |
| Levodropropizine | 28 | – | – | 2 | 11 | 12 | 3 |
| Codeine | 12 | – | – | – | 5 | 6 | 1 |
| Dimemorfan | 9 | – | – | – | 6 | 3 | – |
| Sobrerol | 2 | – | – | 2 | – | – | – |
| Others | 20 | ||||||
| Phenilephrine+chlorphenamine+diphenhydramine | 7 | – | – | 2 | 4 | 1 | – |
| Phenylpropanolamine+clocinizine | 1 | – | – | – | 1 | – | – |
| Promethazine+carbocysteine | 5 | – | – | – | 5 | – | – |
| Pseudoephedrine+dextromethorphan | 4 | – | – | – | 3 | 1 | – |
| Pseudoephedrine+cetirizine | 2 | – | – | – | – | – | 2 |
| Paracetamol+chlorphenamine+dextromethorphan | 1 | – | – | – | – | 1 | – |
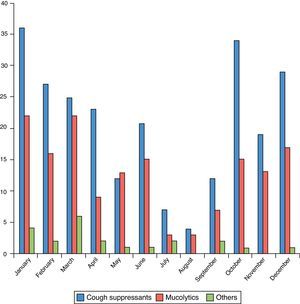
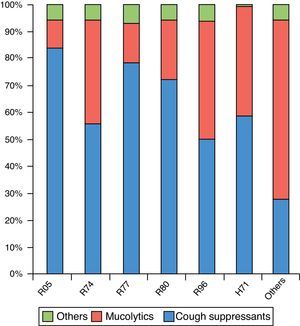
The most frequent diagnosis in this group was R74 (72.8%), followed by R05 (8.7%) (Table 1). The distribution by months and by CIAP-1 codes can be observed in Figs. 1 and 2.
51.9% of the patients who received these drugs were males and 48.1% were females; 27% had a predisposing condition. The average age of the patients was 5 years (1–171 months). 83.1% of the time, these patients were evaluated by a paediatrician and 16.9% by a GP.
Variability of the suitability of the treatmentDescriptive analysisIn the initial analysis that was conducted to assess the suitability taking into account clinical criteria and directions from the data sheet, 85.1% of the prescriptions were deemed unsuitable. These corresponded to 75.1% of the cough suppressants (187), 100% of the mucolytics (155) and 95.5% of the others group (19). Of these drugs, 11.6% were prescribed to children whose age was not identified in the data sheet, essential in children under the age of two (Table 3).
Drugs prescribed under unauthorised conditions in data sheet.
| Drugs | Frequency | ICPC | Reason | FP/PED |
|---|---|---|---|---|
| Mucolytics | ||||
| Ambroxol | 8 | R74 (7), H71 (1) | <2 years | 3/5 |
| Acetylcysteine | 4 | R74 | <2 years | 2/2 |
| Carbocysteine | 2 | H71, R77 | <2 years | 1/1 |
| Cough suppressants | ||||
| Dextromethorphan | 31 | R74 (22), H71 (3), R05 (4), R80 (2) | <1 year (9) 1–2 years (18) 2–6 years (4) | 5/26 |
| Levodropropizine | 2 | H74, R78 | <2 years | 0/2 |
| Others | ||||
| Phenileph+chlorphen+diph | 2 | R74 | <2 years | 1/1 |
ICPC: International Classification of Primary Care; FP: family physician; PED: paediatrician.
In the patients diagnosed with R05 and R74, the treatment was unsuitable in 73 and 82.8%, respectively. There were statistically significant differences when comparing unsuitable treatments in patients diagnosed with R05 (p=0.029) to those not diagnosed with R05; the same happened with those labelled with R74 (p=0.027), and the existence of a higher or lower risk for unsuitable treatment associated with the diagnosis could not be established by logistic regression.
Variability by consultationThere were statistically significant differences between the suitability of the treatments prescribed to patients who were assisted in the doctor's surgery 5 (p=0.006) and those who were assessed in the other locations, but these differences were not confirmed, so we could not establish an association of higher or lower risk of receiving an unsuitable treatment in the doctor's surgery.
Variability by age groupsThere was a statistically significant difference between age groups, children under the age of six and over the age of six (p=0.000). We established a cut-off point at six years old, since this is the limit in which the use of this group of drugs would be considered as a potential alternative. Thus, this treatment was considered unsuitable in children under the age of six in all cases. The analysis by means of logistic regression did not show statistically significant differences.
Variability by type of doctorIf we take into consideration the type of doctor, there was a statistically significant difference between paediatricians and general practitioners (p=0.038); the former are responsible for 83.5% unsuitable prescriptions and the latter for 93.1%, but we could not establish an association of higher or lower risk of receiving inadequate treatment according to the doctor who prescribed the medication.
Variability by drugs or groups of drugsThere were statistically significant differences (p=0.000) when comparing cough suppressants (75.1% are unsuitable) to the other anticatarrhal drugs (99.4%), with a higher percentage of suitable treatments in the group of cough suppressants (CI 95%, 99.3–100%) by means of logistic regression.
DiscussionConsultations for respiratory problems constitute the majority of the day-to-day caseload burden of PC paediatricians; they involve a high morbility rate and represent up to 59.3% of the reasons patients and their families reduce their daily activities.26 This fact is reflected in a study conducted in 2006 in Asturias, where 44.8% of the analysed diagnosis involved the respiratory system and, among these, infections in the upper respiratory tract (R74) was the most frequent diagnosis, representing 23.1% of the total.27 In our study, the most frequent diagnosis was also R74 (42.5%), followed by R96 or asthma/allergic rhinitis (16.9%) (Table 1).
In general, severe respiratory problems are benign and self-limiting; their treatment consists of non-pharmacological and supportive measures. However, there is a great variety of drugs that have been used to try to counteract the symptoms. Their use is not recommended as a general rule due to their uncertain efficacy and the significant risk of toxicity, mostly in children under the age of six.1,28 The accidental ingestion of anticatarrhal drugs in children under the age of four is a frequent reason for consultation due to intoxication in Paediatric Emergencies: close to 75% of these children need treatment and almost half of them have to stay at the hospital for at least a few hours.7 In a study by Morales et al.,8 the R group medications (respiratory system) constituted 26.5% of the total: that is to say, a quarter of the drugs that children take when they attend an Emergency Room. In our study, 16.4% of the prescriptions made were for anticatarrhal drugs, mostly cough suppressants and mucolytics.
On the other hand, a high percentage of the medications used in children, both in Europe and in the United States, are not authorised for use in this age group. According to the data from the European Medicines Agency, in the 1995–2005 period the situation had not improved compared to previous years with regard to the amount of medication with paediatric information, which is approximately one third of authorised medications.8 The use of off-label drugs, that is, when the prescription is made outside what is approved or authorised in the data sheet, is calculated at around 20 and 80% of all prescriptions.29–31 In our study, we analysed the suitability of the prescribed treatments taking into consideration, on one hand, the authorisations in the data sheet, mainly the age, and, on the other hand, the clinical indications. 85% of the anticatarrhal drugs have been prescribed in unsuitable conditions, due to off-label prescriptions in 13.2% of the cough suppressants, 9% of the mucolytics and 10% of the others group. The most frequent cause was age under the authorised limit, which affected children under the age of two. The studies on off-label prescriptions are very heterogeneous in methodology, which hinders the comparison between results, something we observed in our sample and in other studies, such as the one by Olsson et al.,32 where the amount of off-label prescriptions is lower.
In the rest of the cases, the reason was the absence of clinical indication, since anticatarrhal drugs are not indicated in patients with asthma, severe bronchiolitis, severe bronchitis, flu, severe laryngitis, pneumonia, serous otitis media, severe sinusitis and whooping cough.9–24 We must remember that in the cases where there are catarrh symptoms, the use of these drugs could be a valid treatment alternative under medical supervision in selected cases of children over the age of six.25
For these reasons, we pose the question of whether the use of these drugs is suitable within our setting. In this sense, we must remember that in 1985 and for a rational use of medications, the World Health Organisation33 established that it is essential to: (a) prescribe a suitable medication; (b) have it on disposal in a timely manner and at an affordable price; (c) dispense it under the right conditions, and (d) take the prescribed dose during the intervals and time prescribed. Therefore, the right medication has to be effective and of suitable quality and innocuousness.
In our study, the most prescribed subgroup of anticatarrhal drugs was cough suppressants. The distribution was quite similar to the one gathered by Cano Garcinuño et al.1; cloperastine is the leading drug, used for more than half a century, with central and antihistaminic action, of which clinical efficiency in paediatrics has not been adequately studied.5,34 In turn, dextromethorphan is one of the most used for children, as reflected in a survey conducted in the Basque region,34 and it was second in frequency in our sample. In general, it is a safe drug with broad therapeutic windows, but an overdose or toxic ingestion can cause acute side effects in the central nervous system and occasionally even death.35 Its cough-suppressant efficacy has not been proven and it has become a recreational drug for older children and adolescents in many countries.5 Due to the lack of efficacy and the risk of acute adverse effects, the American Academy of Paediatrics does not recommend the use of dextromethorphan in children.36 Codeine, the fourth drug that was prescribed in our study, is considered a “gold standard” in the treatment for cough, although there are no studies that support its use in children, particularly in infants and young children who have a higher risk of toxicity.36 Oral decongestants are vasoconstrictors that act at mucosal level, but they also have systemic effects (cardiovascular and neurological) for which efficacy has not been proven.37 In our study, we registered several products that contained phenilephrine and pseudoephedrine.
When analysing the suitability of the prescriptions, we found that the majority of unsuitable treatments took place in the cases with R05 and R74 diagnosis, compared to the other diagnoses that were gathered; this was probably because they are the most frequent ones in relation to the use of these drugs. Similarly, age was an important factor in these analyses. The highest percentages of unsuitable treatments, as expected, occurred in children under the age of six. A high level of prescriptions for anticatarrhal drugs and its inverse relation to age has been the standard in Spain for at least 30 years, and has been observed both in small-scale studies in offices and in population studies.1,38 Thus, we must be alert in dealing with this age group, where the recommended dose is, mainly, extrapolated from the doses used in adults, without taking into account the differences within this age range regarding physiology, bioavailability and drug toxicity.37
A few previous studies have found differences in the prescription of medications between paediatricians and general practitioners; the first were less inclined to prescribe anticatarrhal drugs in infants.1,8 In our study, the differences in this regard showed a higher percentage of suitable treatments in the case of paediatricians, but without statistically significant differences. In Ontario, a survey conducted among the doctors who assisted a paediatric population reported that 16% of the general practitioners and 4% of the paediatricians recommended anticatarrhal drugs for their patients aged 0–6 months, and this percentage increased to 38% and 14% in children aged 6–12 months.39 A survey conducted in the Basque region showed that, although there are plenty of data advising against the use of anticatarrhal drugs, these drugs were regularly prescribed by paediatricians; 67.5% of the respondent paediatricians prescribed cough suppressants regularly.34
At the same time, we found differences when analysing cough suppressants compared to mucolytics/others. There were more suitable treatments in the first group than in the second one, which could be explained by the absence of indication in the cases in which drugs from the mucolytic/others group were used and the possibility in some cases of accepting as therapeutic alternative cough suppressants in children over the age of six.
Our study has limitations, in spite of having selected a random sample. The size of the sample may or may not be representative of the prescriptions of anticatarrhal drugs made, but we consider that it does reflect the current situation, in which the use of these drugs is frequent in spite of their low efficacy and potential toxicity. The strength of our study is based on the gathering of prescription data through clinical records, and associated with a clinical diagnosis.
To conclude, we must emphasise that it is important that children only receive drugs of proven efficacy and with a favourable risk–benefit ratio. To avoid unsuitable prescriptions, apart from promoting education to convince paediatricians themselves, we must work with the parents, letting them know the real efficacy and the potential side effects of these drugs, so that a joint doctor–parent decision that is personalised for each case can be made. The doctor's predisposition should be to not recommend this type of drugs, based on current available data.40 There might be cases in which the parents want, in spite of everything, their child to receive some kind of medication. In this situation, the paediatrician should resort to those drugs that have a better security profile.40
Conflict of interestsThe authors declare that there are no conflicts of interest.
The authors thanked all the PC paediatricians of the Area V from the Sanitary Service of the Principado de Asturias for their daily work, availability and support in the performance of this study.
Please cite this article as: Suárez-Castañón C, Modroño-Riaño G, López-Vilar P, Martínez-Blanco J, Iglesias-Cabo T, Solís-Sánchez G. Uso de anticatarrales en menores de 14 años en consultas de Atención Primaria. An Pediatr (Barc). 2016;84:10–17.