To review the current scientific evidence on the efficacy of universal vitamin D supplementation in healthy children with no risk factors and to propose updated modifications to the recommendations provided in the main clinical practice guidelines.
MethodsScoping review through a literature search conducted in PubMed for articles published in English or Spanish in the past 15 years using the following MeSH search terms: (vitamin D) AND (supplementation). The search yielded 2133 articles, of which 22 were selected.
ResultsIn infants aged less than 1 year without risk factors, administration of 400 IU/day improves serum levels of calcifediol, but most studies have not found an association with improved bone health assessed by dual-energy X-ray absorptiometry. In children aged more than 1 year with calcifediol levels of less than 35 ng/mL, supplementation with 400 to 800 IU/day of vitamin D decreases the risk of respiratory infection. There is still no evidence in the pediatric population that vitamin D supplementation either decreases the risk or severity of other infections or offers any other clinically significant extraskeletal benefits.
ConclusionBased on the main clinical practice guidelines, supplementation with vitamin D at a dose of 400 IU/day is recommended for breastfed infants aged less than 1 year or infants who do not achieve the recommended daily intake through infant formula. In children aged more than 1 year, supplementation should be individualized.
Revisar la evidencia científica disponible sobre la eficacia de la suplementación universal con vitamina D en población pediátrica sana, sin factores de riesgo, y actualizar posibles modificaciones en las recomendaciones de las principales guías de práctica clínica.
MétodosRevisión de alcance mediante búsqueda bibliográfica en PUBMED durante los últimos 15 años, utilizando los siguientes términos de búsqueda MeSH: (vitamin D) AND (supplementation) en inglés o español. Se obtuvieron 2133 artículos de los que finalmente se seleccionaron 22.
ResultadosEn niños sin factores de riesgo menores de 1 año, la administración de 400 UI/día mejora los niveles séricos de calcidiol, sin embargo, en la mayoría de los estudios no se relaciona con una mejoría en la salud ósea valorada por densitometría. En mayores de 1 año con niveles de calcidiol < 35 ng/mL, la administración de entre 400 y 800 UI al día de vitamina D disminuye el riesgo de padecer una infección respiratoria. En niños aún no se ha demostrado que la suplementación con vitamina D disminuya el riesgo ni la gravedad de otros procesos infecciosos, ni suponga una mejoría clínica para el resto de las funciones extra esqueléticas.
ConclusionesSegún las principales guías de práctica clínica, se recomienda suplementar con 400 UI/día de vitamina D a los niños menores de un año que reciban lactancia materna o que no alcancen la ingesta diaria recomendada a través de la fórmula infantil adaptada. En los mayores de 1 año, la suplementación debe ser individualizada.
In recent years, there has been an evident and growing interest in vitamin D, largely due to the discovery of its extra-skeletal functions. However, despite advances in knowledge and an exponential increase in scientific publications, the evidence on universal vitamin D supplementation in children is limited and the recommendations of clinical practice guidelines have hardly changed in the past few decades.1 The latest updates from the Committee on Nutrition of the Asociación Española de Pediatría (AEP) and the European Society of Pediatric Gastroenterology, Hepatology and Nutrition were published in 20122 and 2013,3 respectively. This is a topic that continues to be the subject of considerable debate in scientific societies.4
Few foods are natural sources of vitamin D. The main dietary sources are fatty fish, liver and egg yolks, in addition to fortified products such as milk and cereal. However, the amounts provided by these foods are often insufficient to reach the recommended daily allowance: 400 IU in infants aged less than 1 year and 600 IU in children aged 1 to 18 years.5
Our objective was to provide updated knowledge on the need for vitamin D supplementation and monitoring in healthy children, and to propose hypotheses to shed light on the reason why many experimental studies have failed to demonstrate the efficacy of supplementation with this vitamin that has hormonal activity and plays such an important role in the functioning of the human body.
Material and methodsTo review the available evidence on the efficacy of universal vitamin D supplementation in the pediatric population, we conducted a scoping review by means of a literature search in PubMed for meta-analyses and clinical trials published in the last 15 years (date of the search: June 1, 2024). We used the following terms ("Vitamin D" [MeSH Terms] AND ("supplemental" [All Fields] OR "supplementating" [All Fields] OR "supplementation" [All Fields] OR "supplementation s" [All Fields] OR "supplementations" [All Fields] OR "supplementation" [All Fields] OR "supplemention" [All Fields]) AND ((2009/6/1:2024/6/1[pdat]) AND (english[Filter] OR spanish[Filter]) AND (allchild[Filter])), which yielded 2133 articles, of which 22 were finally selected (13 clinical clinical trials and 9 meta-analyses). The supplemental material provided in Appendix B (flow diagram and Tables 1S–3S) presents the selection process, selected articles and the standards applied based on the PRISMA guidelines. Table 1 summarizes the results of the selected clinical trials. In addition to the scoping review, we reviewed the main clinical practice guidelines currently in use to describe the most recent recommendations of the different scientific societies
Clinical trials related to the efficacy of vitamin D supplementation in healthy pediatric populations in terms of bone health and the risk of respiratory infections.
| Author and year | Design | Population | Age | Intervention | Baseline calcifediol | Outcomes |
|---|---|---|---|---|---|---|
| Bone health | ||||||
| Gharibeh N, 2023. Canada | Double-blind randomized clinical trial without placebo control | Healthy breastfed infants in both groups (100% at month 1, 93% at month 3, 85-83% at month 6 and 44-40% at 1 year) | 1 month | 400 (n = 49) vs 1000 (n = 49) IU of vitamin D3 per day for 11 months | All <20 ng/mL | At one year of life: no differences in bone density or bone resorption markers, calcifediol greater in the 1000 IU/day group. |
| Gallo S, 2016. Canada | Double-blind randomized clinical trial without placebo control | Healthy breastfed children | 1 month | 400 (n = 25) vs 800 (n = 25) vs 1200 (n = 26) vs 1600 (n = 11) IU/day vitamin D3 for 11 months | Mean, 22.44 ng/mL (6% <12 ng/mL) | At 3 years of life: no differences in bone density or serum levels. |
| Gallo S, 2013. Canada | Double-blind randomized clinical trial without placebo control | Healthy breastfed children | 1 month | 400 (n = 39) vs 800 (n = 39) vs 1200 (n = 38) vs 1600 (n = 16) IU/day vitamin D3 for 11 months | Mean between 20.3 and 24.8 ng/mL | At 12 months of life: equal progressive improvement in bone density in all groups. |
| Lin CH, 2022. Taiwan | Double-blind randomized placebo-controlled trial | Healthy breastfed children | 10 días | 400 IU per day of vitamin D3 (n = 37) vs placebo (n = 35) for 4 months | All > 10 ng/mL Mean of 18.7 in the vitamin D group vs 19.6 in the placebo group | At 4 months: increased vitamin D levels, decreased PTH levels and no differences in bone mineral density in the treatment group. Placebo group: 45% with calcifediol < 10 ng/mL |
| Middelkoop K, 2024. South Africa | Double-blind randomized placebo-controlled trial | Black children without HIV infection | 6-11 years | 10 000 IU vitamin D3 weekly (n = 228) vs placebo (n = 222) for 3 years | Vitamin D 28.2 ng/mL vs placebo 27.9 ng/mL | After 3 years: increased vitamin D levels, no differences in bone density or number of fractures. |
| Ganmaa D, 2024. Mongolia | Double-blind randomized placebo-controlled trial | Children in 18 public schools | 6-13 years | 14 000 IU per week vitamin D (n = 4176) vs placebo (n = 4172) for 3 years | 95.5% <20 ng/mL | No difference in the number of fractures at 3 years of follow-up. |
| Rosendahl J, 2018, Helsinki | Double-blind randomized clinical trial without placebo control | Newborns (breastfed > 85%) | 2 weeks | 14 000 IU per week vitamin D (n = 4176) vs placebo (n = 4172) for 3 years | 95% >20 ng/mL | At 2 years, no differences in bone mineral density (tomography), higher calcifediol levels in group managed with 1200 IU. |
| Respiratory infections | ||||||
| Ganmaa D, 2020. Mongolia | Randomized, double-blind placebo-controlled trial | Children from 18 public schools | 6-13 years | 14 000 IU per week vitamin D (n = 4418) vs placebo (n = 4433), for 3 years | Mean, 11.9 ng/mL | No differences in TB infections or admission for respiratory infection at 3 years of follow-up |
| 95.4% <20 ng/mL | ||||||
| Loeb, 2019. Vietnam | Randomized, double-blind placebo-controlled trial | Healthy children in a rural district | 3-17 years | Vitamin D: 14 000 IU D3 per week (n = 650) vs placebo (n = 650), for 8 months | 26.3 ng/mL in vitamin D group vs 26.1 ng/mL in placebo group | Vitamin D group: decrease in HR for viral respiratory infections (0.81; 95% CI, 0.66-0.99), but not for influenza infection |
| Rosendahl J, 2018. Helsinki | Double-blind randomized clinical trial without placebo control | Newborns (breastfed > 85%) | 2 weeks | 400 (n = 489) vs 1200 (n = 486) IU vitamin D3 per day for 24 months | 95% >20 ng/mL | At 2 years, no differences in respiratory infections reported by parents |
| Aglipay M, 2017. Canada | Double-blind randomized clinical trial without placebo control | Healthy children | 1-5 years | 400 (n = 354) vs 2000 (n = 349) IU vitamin D3 per day for 4 months between September and May | 36.9 (400 IU) vs 35.9 (2000 IU) | At 4 months, no differences in clinical or microbiological diagnosis of respiratory infections nor the time elapsed to the first infection. |
| Huang YN, 2022. Taiwan | Randomized, double-blind placebo-controlled trial | Preschool-age children attending kindergarten | Mean, 4 years | Placebo (n = 113) vs 2000 IU vitamin D3 per day for 1 month | Not available | At 6 months, decrease in influenza that was not significant (P = .095) without changes in enterovirus infection |
| Urashima M, 2010. Japan | Randomized, double-blind placebo-controlled trial | Children (70% healthy, 30% with underlying disease) | 6-15 years | Placebo (n = 213) vs 1200 IU vitamin D3 per day for 4 months | Not available | Reduction in influenza infection (RR, 0.58; 95% CI, 0.34–0.99; P = .04) |
| Manaseki-Holland S, 2012. Kabul | Randomized, double-blind placebo-controlled trial | Infants | 1-11 months | Placebo (n = 1522) vs 100 000 (n = 1524) IU every 3 months for 18 months | Not available | No difference in the first episode of pneumonia. Higher risk of recurrent pneumonia in the vitamin D group |
HIV, human immunodeficiency virus; HR, hazard ratio; TB, tuberculosis.
According to the latest Cochrane review on the subject6 and other recent meta-analyses,7 supplementation with 400 IU/day in infants aged less than 1 year has been found to be safe and effective in improving vitamin D status and decreasing the percentage of infants with calcifediol levels below 20 ng/mL. However, this improvement in serum levels is not always associated with an improvement in bone health assessed by means of bone density scans. One of the main limitations is the lack of well-designed studies, as only three clinical trials comparing the use of vitamin D against placebo have been published in infants, all of them with a very small number of patients.
The most severe form of vitamin D deficiency is rickets, the incidence of which has been increasing in recent years in Europe, mainly in at-risk groups. Observational studies have shown that the use of vitamin D supplementation, as well as the use of fortified foods as a public health policy, reduces the prevalence of rickets.8,9 There is also evidence from large case series of healthy infants who received prophylaxis with 400 UI/day in which there were no diagnosed cases of rickets.10
There have been several clinical trials comparing the administration of various doses of vitamin D in the first year of life (from 400 to 2000 IU/day), although they were dose-response studies without comparison with placebo. They showed that administration of doses greater than 400 IU/day did not provide any benefit in terms of body composition, gross motor skills or bone density at 3 years of follow-up.11,12 In a recent trial, a group of healthy breastfed infants with calcifediol levels below 20 ng/mL at age 1 month were randomly assigned to receive 400 or 800 IU/day. At 12 months of follow-up, there were no differences in bone health assessed by means of bone density scans or in serum calcifediol levels between the two groups. These studies show that, in healthy infants, supplementation at doses greater than 400 IU/day does not seem to provide additional benefits, and that, in the absence of risk factors, serum levels should not be monitored, given that, regardless of the calcifediol level, a dose of 400 IU/day seems sufficient to achieve the nutritional target for adequate bone health in this age group.
Bone health in children aged 1 to 18 yearsIn this age group, there is even less evidence. In a recent published meta-analysis, vitamin D supplementation for one year in children with calcifediol levels greater than 8 ng/mL has a minimal effect on the bone mineral density of the hips in densitometry, increasing 6.8 mg/cm2 (95% CI, 0.7-1.9),13 without evidence of changes in any of the other regions. In consequence, the authors did not support universal vitamin D supplementation, although they cautioned that the results could not be extrapolated to children with vitamin D levels outside the studied range. There is also no evidence that supplementation is effective in reducing the incidence of fractures in this age group.
Extraskeletal healthOne of the extraskeletal benefits for which there is the most evidence is its role in antimicrobial activity: several clinical trials and meta-analyses14 have shown that supplementation with vitamin D can, in certain circumstances, reduce the risk of developing respiratory infections (Table 1). One of the most recent meta-analyses15 showed that, vitamin D supplementation reduced the overall risk of respiratory infection (OR, 0.92; 95% CI, 0.86-0.99) and was most effective in patients aged 1 to 18 years who received low doses of vitamin D (between 400-800 IU/day), with no underlying disease and with calcifediol levels of less than 35 ng/mL (OR, 0.56; 95% CI, 0.38-0.82). Considering that very heterogeneous methods were used for the definition of the different types of respiratory infection (otitis, pneumonia, upper respiratory tract infection…), that the improvement was limited to a specific group and that the magnitude of the effect was low (OR, 0.9; CI of almost 1), these results still seem insufficient to recommend universal vitamin D supplementation.
There is no evidence that vitamin D supplementation decreases the incidence or severity of other types of infection. Moreover, studies in developing countries have shown that the use of high-dose vitamin D supplementation in malnourished infants increases the risk of recurrent pneumonia.16 These findings could be due to the sharp rises and falls in vitamin D levels, which can suppress adaptive responses to infection, boost innate responses and block the activity of enzymes that synthesize and catabolize calcitriol in extrarenal tissues. This could result in reduced concentrations of this active metabolite in disease foci. There is also no evidence that vitamin D supplementation in healthy pediatric population decreases the risk of atopy, autoimmune diseases or cancer, among other diseases.17,18
Main clinical practice guidelinesVitamin D supplementationIn infants (age < 1 year), nearly every guideline recommends universal supplementation with 400 IU a day of vitamin D, independently of infant feeding modality.3,19–24 Foregoing supplementation is only considered in the small group of infants who reach a daily intake of at least 1 liter of adapted infant formula (equivalent to 400 IU/day).1,2 These recommendations are mainly based on the impossibility of reaching the recommended daily intakes through diet, the low sun exposure in this age group, the role of vitamin D supplementation as a public health strategy for prevention of rickets end, evidence from multiple safety studies supporting the use of this dose25 and the ethical issues involved in conducting a placebo controlled trial in infants, chiefly in relation to those who are breastfed.
In children aged more than 1 year, most guidelines do not recommend universal supplementation, but individualization based on the presence of risk factors, sun exposure and dietary vitamin D intake. Only specific countries in the northern hemisphere recommend universal supplementation or supplementation during the winter months (Table 2).
Recommendations from leading scientific societies on vitamin D supplementation in pediatric patients by age group (age < and > 1 year).
| Scientific societies | Recommendations for infants (age < 1 year) |
|---|---|
| AEP (2012), AAP (2008), Polish Society of Pediatric Endocrinology (2018) | Breastfed infants: 400 IU/day. Infants with daily intake of less than 1 liter of infant formula: 400 IU/day. |
| ESPGHAN (2013), European Academy of Paediatrics (2017), Indian Academy of Pediatrics (2021), Italian Society of Pediatrics (2018) | Universal: 400 IU/day |
| French expert committee (2022) | Universal: minimum of 400 IU/day and maximum of 800 IU/day |
| Scientific societies | Recommendations for children aged 1-18 years |
|---|---|
| AAP (2008) | Risk groups if RDA not met (600 IU/day) |
| Italian Society of Pediatrics (2018) European Academy of Paediatrics (2017), ESPGHAN (2013), APED (2012), Indian Academy of Pediatrics (2021) German Society of Pediatrics (2019) | 400-1000 IU/day in risk groups |
| Department of Health of United Kingdom (2016) | Universal supplementation with 400 IU/day during winter months |
| French expert committee (2022) | Universal supplementation year round with 400-800 IU/day. Double dose for risk groups. |
Abbreviations: AEP, Asociación española de Pediatría (Spanish Association of Pediatrics; AAP, American Academy of Pediatrics; ESPGHAN, European Society for Paediatric Gastroenterology, Hepatology and Nutrition; RDA, recommended daily allowance.
n spite of the heterogeneity of the recommendations on vitamin D supplementation (mainly in children aged more than 1 year), all guidelines are against monitoring vitamin D levels in healthy children without risk factors who are asymptomatic. The strategy has not been found to be cost-effective, and in addition to the scarce evidence on supplementation in this population, there is no consensus on the cut-off points for defining deficiency. All of this can lead to inadequate prescription of vitamin D supplementation and unnecessary blood tests.26 The main scientific societies agree in considering that measurement of calcifediol levels is only indicated in select cases (Table 3). In children who require monitoring, measurement in the last months of winter is recommended.
Indications for measurement of blood calcifediol levels in pediatric patients.
| SYMPTOMATIC |
|
| RISK GROUP |
|
| CHRONIC DISEASES |
|
Abbreviation: ARFID, avoidant/restrictive food intake disorder.
In Spain, the adherence to clinical practice guidelines is poor,27 and, based on recent studies conducted in Europe and the United States, adherence in some countries is below 15%. To address this issue, some health care organizations have implemented a series of measures to try to improve their performance (promoting training of health care professionals, prescribing in the labor and delivery ward with provision of written information for families on the importance of supplementation, outpatient monitoring of adherence through electronic prescribing systems…).28
Vitamin D toxicityIn most of the cases of vitamin D toxicity reported in the literature, children received very high doses (total cumulative intake over days or weeks ranging from 240 000 to 4 500 000 IU).29 A recent meta-analysis that included 32 clinical trials in patients aged 0 to 6 years who received between 1200 and 10 000 IU/day found no differences in the number of serious adverse events (death or hospital admission) compared to children who received lower doses.30 However, it is important to be cautious, as there is a subset of children with specific polymorphisms or carrying CYP24A1 variants who are at increased risk of developing hypercalcemia and vitamin D toxicity at lower doses. In addition, an excess of vitamin D could have a negative impact on bone mineralization during childhood and adolescence, which are considered critical periods for bone health.
The established tolerable upper intake level (UL) varies between scientific societies; thus, the European Food Safety Authority (EFSA) has set it at 1000 IU/day for infants and 2000 IU/day for children aged 1 to 10 years, while the Institute of Medicine (IOM) has set it at 1000 IU/day for infants aged less than 6 months and 1500 IU/day for infants aged 6 to 12 months.5,31 No cases of toxicity have been reported in association with smaller doses, so the recommendation for supplementation with 400 IU/day in infants up to 1 year of age is completely safe and does not require lowering the dose of vitamin D supplementation as dietary intake increases in the infant.
Notwithstanding, in recent years there has been an increase in the incidence of vitamin D toxicity32 that can probably be attributed to the increased frequency of prescribing and the commercialization of vitamin D supplements at widely varying concentrations that may give rise to administration errors. In consequence, it is recommended that directions always be given in writing, including the timing, preparation, concentration and dose, in addition to verifying during primary care visits that supplementation is being administered correctly. The use of electronic prescribing systems may be useful to check on the number of packages ordered and dispensed to the patient.
DiscussionExcept in infants aged less than one year, the evidence to date is insufficient to support universal vitamin D supplementation in the healthy pediatric population as an effective measure for maintaining good health. Few clinical trials have been conducted, and most of the studies in the literature have methodological limitations.
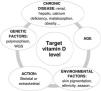
Administration of vitamin D alone for the purpose of improving bone health and/or extraskeletal benefits may be overly optimistic, since they depend on multiple factors (nutritional factors such as calcium, phosphate or magnesium levels, lifestyle habits, physical activity, genetics, race, endocrine factors…). We must also keep in mind the importance of sun exposure (through the effects of UVB radiation) as a catalyst of vitamin D synthesis, as well as the fact that vitamin D status depends on multiple additional factors. Obesity, medication, genetic factors, chronic diseases or disease in the months following childbirth play an important role in maternal vitamin D status, and all these factors should be taken into account in the design of clinical trials, which is not always the case.
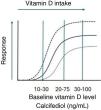
On the other hand, as with any other nutrient, the magnitude of the effect of a given intervention or supplementation strategy will depend on the baseline status (Fig. 1: dose-response graph); therefore, information on vitamin D status is necessary to correctly interpret the results of clinical trials. However, determining whether a healthy child has vitamin D deficiency or insufficiency may be challenging because calcifediol, despite its benefits, also carries limitations due to its long half-life (2-3 weeks) and its greater stability compared to other metabolites. In fact, previous evidence in children has demonstrated the lack of a linear relationship between calcifediol and most outcome variables in this age group, and this, among other reasons, has led to the variation in the cut-off points established by different scientific societies. When it comes to bone health, the “lowest” threshold is 10 to12 ng/mL, since most pediatric patients with rickets have levels below 10 ng/mL; however, cases in patients with higher values have also been reported.33 In adults, below the threshold of 20 ng/mL, decreasing concentrations of calcifediol give rise to an abrupt increase in parathyroid hormone levels; but we do not know the corresponding value in pediatric patients (it may be even lower),34 which evinces that this association depends, among many other factors, on age, although also on the duration of the deficiency. Yet higher levels of vitamin D seem necessary to achieve extraskeletal benefits, but the evidence on the subject is even more scarce. Therefore, rather than universal cut-off points, we should aim to establish a vitamin D status target that will likely be different for each patient or individual depending on their age, genetics, race, diet, underlying disease, risk factors, calcium levels, and outcome of interest, whether it involves bone or extraskeletal health (Fig. 2).
Informational graphic on the intake-response curve for a nutrient showing the efficacy of supplementation with the same dose of vitamin D depending on the baseline concentration of the nutrient.
Note: the exact shape of the curves and the values of the cut-off points have not been established.
Adapted from: Heaney RP. N Engl J Med. 2012;367(1):77-78.
In the past few years, efforts have been made to advance scientific knowledge on novel markers, and, based on the “free hormone hypothesis”, the fraction of vitamin D not bound to proteins (free 25-hydroxyvitamin D) would be the bioavailable fraction allowing the vitamin to produce its biological effects and therefore the marker that can best reflect vitamin D status. This hypothesis seems to be most applicable when protein and/or albumin levels are low (cirrhosis, pregnancy, undernutrition…).35 However, in the healthy pediatric population, the correlation between total calcifediol levels and free calcifediol levels is linear and robust, so measurement of free calcifediol levels does not seem to offer significant benefits compared to measurement of total calcifediol levels.36
Genetic factors are becoming increasingly important, and four groups of single nucleotide polymorphisms have been described in genes involved in the synthesis and transport of the active forms of vitamin D (hydroxylation, synthesis, transport) in addition to the gene that encodes the vitamin D receptor (VDR) present in different tissues. These variants determine the greater or lesser affinity of the vitamin D-binding protein (VDBP) for vitamin D and its receptors, which could explain up to 10% of the variance in calcifediol levels37; the response to a dose of vitamin D varies depending on the variant,38 and some variants are associated with or may constitute risk factors for certain diseases.39 Recently, in some chronic diseases, whole genome sequencing (WGS) has been used to develop several polygenic risk scores associated with vitamin D status and to the response to supplementation with this vitamin.40
The data on the prevalence of vitamin D deficiency in the pediatric population of Spain is scarce. In addition, factors such as geographical differences in sun exposure and variation in dietary habits further complicate the interpretation and comparison of the existing evidence. This lack of knowledge limits the capacity to develop effective strategies regarding the need for supplementation.
ConclusionsAt present, there is limited evidence on vitamin D supplementation in the healthy pediatric population.
The main clinical practice guidelines recommend supplementation with 400 IU/day of vitamin D in infants aged less than 1 year who are breastfed or whose intake through infant formula does not reach the recommended daily allowance. In children aged more than 1 year, supplementation should be individualized.
Measurement of blood calcifediol levels is not recommended in children without risk factors who are asymptomatic.
Vitamin D should be prescribed specifying the dose to be administered and the duration of treatment. In addition, its correct administration should be verified periodically during routine check-up visits.
In Spain, there have been few well-designed experimental or cohort studies on vitamin D supplementation in healthy children. Taking into account the importance of factors such as sun exposure and genetics, we must advocate for research to be conducted on the subject to enable the development of appropriate recommendations adapted to our region.
FundingThis research did not receive any external funding.
The authors have no conflicts of interest to declare.