Congenital heart defects (CHDs) are the most prevalent and severe type of major congenital anomalies (CAs). The objective of this study was to determine the frequency and distribution of CHDs in the Valencian Region from 2007 to 2014, describing common characteristics of the patients and their mothers.
Material and methodsWe retrieved data on CHDs in live births, stillbirths and cases of termination of pregnancy for foetal anomaly between 2007 and 2014 (codes Q20–Q26 in the 10th Revision of the International Classification of Diseases-British Paediatric Association, ICD10-BPA) from the population-based registry of congenital anomalies of the Valencian Region. We calculated the prevalence per every 10000 births of CHDs overall and by subtype, analysed temporal trends and the geographic distribution of cases, and documented the presence of associated noncardiac malformations.
ResultsWe identified 3671 cases of CHD, corresponding to 38.6% of all CAs. The prevalence was 91.1/10000 (IC 95%: 88.1–94.0) with a predominance of septal defects, chiefly atrial septal defect (48.5/10000; IC 95%: 46.4–50.6) and ventricular septal defect (36.1/10000; IC 95%: 34.3–38.0). We found the highest prevalence in the province of Castellon (137.8/10000; IC 95%: 127.5–148.1). The sex distribution was 47.3% male and 44.3% female. Of all cases, 90.9% corresponded to live births and 65.6% were diagnosed at birth. The most frequent associated extracardiac malformations were musculoskeletal, and 19.2% of patients had syndromes. The most frequent maternal diseases were diabetes mellitus, hypothyroidism and urinary tract infections.
ConclusionsThe prevalence of CHD and atrial septal defects was higher compared to European data, while the prevalence of ventricular septal defects was similar. Musculoskeletal malformations were the noncardiac CAs most frequently associated with CHDs.
Las anomalías congénitas cardíacas (ACC) son el tipo de anomalías congénitas (AC) mayores de más prevalencia y gravedad. El objetivo fue determinar la frecuencia y distribución de las ACC en la Comunitat Valenciana desde 2007 hasta 2014, describiendo las características comunes de los pacientes y sus madres.
Material y métodosSe seleccionaron del Registro poblacional de AC de la Comunitat Valenciana los pacientes con ACC nacidos vivos, nacidos muertos e interrupciones voluntarias del embarazo entre 2007-2014 (códigos Q20-Q26 de la Clasificación Internacional de Enfermedades 10.ª Revisión, Asociación Pediátrica Británica). Se calculó la prevalencia por 10.000 nacidos para el total de ACC y sus subtipos, se describió su evolución temporal y distribución geográfica, y se identificaron las malformaciones no cardíacas asociadas.
ResultadosSe identificaron 3.671 pacientes con ACC representando el 38,6% de las AC. La prevalencia fue 91,1/10.000 (IC95%:88,1-94,0) destacando especialmente la comunicación interauricular (48,5/10.000 [IC95%:46,4-50,6]) y la comunicación interventricular (36,1/10.000 (IC95%: 34,3-38,0)). La provincia de Castellón obtuvo la prevalencia más alta (137,8/10.000 [IC95%:127,5-148,1]). El sexo de los pacientes fue 47,3% niños y 44,3% niñas. El 90,9% de los pacientes nacieron vivos y el 65,6% fueron diagnosticados al nacimiento. Las malformaciones asociadas a las ACC más frecuentes fueron las musculoesqueléticas y el 19,2% tenían síndromes asociados. La diabetes mellitus, hipotiroidismo e infecciones del tracto urinario fueron las principales patologías maternas.
ConclusionesLas prevalencias de ACC y comunicación interauricular identificadas son superiores a las europeas, mientras que la de la comunicación interventricular es similar. Las malformaciones musculoesqueléticas fueron las AC no cardiacas más relacionadas con las ACC.
Congenital anomalies (CAs) are defined as structural and functional disorders developed during pregnancy that can be diagnosed prenatally, at birth or during childhood.1
This year, the World Health Organization highlighted the importance of CAs in one of their reports, identifying them as the cause of around 5.0% of neonatal deaths worldwide as well as 8.0% of postneonatal deaths.2 It is estimated that severe CAs are diagnosed in approximately 3 million children each year, a number that explains the 303000 newborns that die within four weeks from birth due to this type of anomalies.1,3
Congenital anomalies are responsible for 25.0% of infant deaths in Europe, with a prevalence that, according to the data collected between 2010 and 2014, reaches 248.6 cases per 10000 births.4,5
The most recent prevalence data published in Spain remains similar to European data, but with a slight decline in the frequency of live births (LBs) from 2.2% to 1.4%. This change is due to the improvement in prenatal diagnosis techniques, which is directly associated with the increased frequency of termination of pregnancy for foetal anomaly (TOPFA), a trend that has also been observed in other developed countries.6,7
Congenital heart defects (CHDs) are the main type of CA, amounting to 28.0% of prenatal malformations with a prevalence of approximately 8 cases per 1000 live births.8,9 Of the 51546 CAs documented by the European Surveillance of Congenital Anomalies (EUROCAT), 16790 were CHDs, representing 32.6% of the total, which evinces the magnitude of this problem.4
In recent years, the prevalence of CHD has increased due to advances in diagnostic techniques, an increasing trend in maternal age and the and longer survival of affected individuals, which makes transmission of the defect to the offspring possible and increases the risk of recurrence.10,11
Congenital heart defects are also relevant due to the associated risks, with leading causes of morbidity and mortality associated with CAs.12 EUROCAT estimates that CHDs account for 16.6% of foetal deaths due to CAs after 20 weeks of gestation. This percentage reaches 30.6% if we add neonatal deaths in the first week of life.13
The objective of our study was to establish the frequency and distribution of CHDs in the Valencian Region (VR) between 2007 and 2014, describing the main characteristics of the patients and their mothers.
Materials and methodsWe conducted a cross-sectional, observational epidemiological study using data retrieved from the Population-Based Congenital Anomaly Registry of the Valencian Region (PCAR-VR).
The cases in the PCAR-VR are identified by searching the Public Spanish Hospital Discharge Dataset registry, the Perinatal Mortality Registry of the VR and the Registry of TOPFA of the VR. The Metabolic Disorders Registry of the VR is used a supplementary source for data on deliveries, newborns and mothers. Based on the data found in these sources, clinical records are reviewed (from hospitals and primary care clinics) for the first year of life, allowing confirmation of cases of CAs and the collection of clinical and epidemiological information. Once confirmed, cases are added to the PCAR-VR with the pertinent codes of the 10th Revision of the International Classification of Diseases-British Paediatric Association (ICD10-BPA).
For our study, we selected the LBs, stillbirths (SBs) and TOPFAs in mothers who were residents of the VR from the 2007 to 2014 period that listed at least 1 CHD code (codes Q20–Q26 in the ICD10-BPA) from the PCAR-VR. Cases in this registry are entered according to the protocol formulated and agreed upon by EUROCAT. For example, cases of minor CAs such as patent or persistent foramen ovale are only included in the PCAR-VR if they are associated with a major CA, and cases of ostium secundum atrial septal defect are only included if the defect is still present 6 months after birth.
We collected the following variables for each case: sex of the patient, type of diagnosis, province of residence, hospital where the defect was diagnosed, maternal nationality, relevant medical history of the mother, maternal gestational disease and maternal drug consumption during pregnancy. We also documented survival in days, birth weight, gestational age at birth, type of CHD, number of CHDs per case and associated CAs or syndromes.
We calculated the overall and annual prevalence of CHDs with their 95% confidence intervals (CIs) to analyse temporal trends in the 2007–2014 period.
We also conducted an analysis by province to describe geographical variations in CHD in the VR, studying the distribution in the 3 provinces and calculating the corresponding prevalences and 95% CIs, using the births from 2007 to 2014 in each province of the VR as reference.
We classified CHDs by subtype and calculated the frequency for each. We then calculated the overall and annual prevalence and their 95% CIs for the most frequent subtypes to describe their temporal trends.
We analysed the distributions of sex, type of delivery (LB, SB and TOPFA) and time of diagnosis (prenatal, at birth, after birth) over the 8-year period. In addition, we counted and grouped cases by birth weight (low <2500g, normal 2500–4000g and overweight >4000g) and gestational age at birth (very preterm <32, preterm 32–36, normal 37–40, post-mature >40 weeks) to describe the distribution of these variables.
Mothers were classified by age, nationality, personal medical history, diseases developed during pregnancy and drugs consumed during pregnancy to identify the most frequent maternal characteristics in cases of CHD.
Last of all, we analysed the noncardiac CAs (coded with a code outside the Q20–Q26 interval in the ICD10-BPA) associated with the CHDs, calculating their frequency in this group to identify the malformations most commonly associated with cardiac defects.
ResultsThe PCAR-VR registered 3671 cases of CHD between 2007 and 2014, which amounted to 38.6% of the CAs diagnosed during this period, with a prevalence of 91.1 cases per 10000 births (95% CI, 88.1–94.0). This prevalence remained stable throughout the studied period except in 2013 and 2014, when the prevalence increased to 99.9/10000 (95% CI, 90.6–109.2) and 104.4/10000 (95% CI, 94.9–113.8), respectively (Table 1).
Number of cases and prevalence of cardiac heart defects in the Valencian Community between 2007 and 2014.
| Year | Cases (n) | Prevalence (per 10000a) | Confidence interval (95%) |
|---|---|---|---|
| 2007 | 477 | 86.6 | (78.9, 94.4) |
| 2008 | 527 | 91.1 | (83.4, 98.9) |
| 2009 | 456 | 86.1 | (78.2, 93.9) |
| 2010 | 468 | 90.5 | (82.3, 98.6) |
| 2011 | 419 | 84.1 | (76.1, 92.1) |
| 2012 | 421 | 89.0 | (80.6, 97.5) |
| 2013 | 438 | 99.9 | (90.6, 109.2) |
| 2014 | 465 | 104.4 | (94.9, 113.8) |
| Total | 3671 | 91.1 | (88.1, 94.0) |
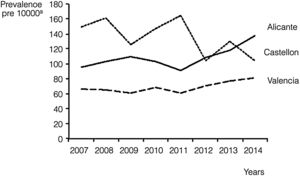
We also calculated the overall and annual prevalence for each province, with the highest prevalence found in Castellon, with 137.8 cases per 10000 births (95% CI, 127.5–148.1), followed by Alicante, with 107.6 cases per 10000 births (95% CI, 102.2–112.9). Nevertheless, there was a progressive increase in prevalence in Alicante during the period under study, as it reached 137.6 cases per 10000 (95% CI, 119.5–155.7) in 2014, surpassing the prevalence in Castellon in the same year (104.5/10000; 95% CI, 77.2–131.7). Valencia remained in the third position during the whole period, with an average prevalence of 68.4 cases per 10000 births (95% CI, 64.9–71.9) and troughs in prevalence of 60.8/10000 (95% CI, 51.6–70.0) in years 2009 and 2011 (Fig. 1).
The proportions of cases of CHD in male versus female individuals were very similar (47.3% boys and 44.3% girls). The 8.4% of cases where the sex was unknown corresponded to instances of TOPFA in which this variable had not been documented. When it came to the type of delivery, in addition to the 8.4% cases of TOPFA, 90.0% of CHDs corresponded to LBs and 0.7% to SBs. Of all cases of CHD, 65.6% were diagnosed at birth, 21.3% prenatally and 12.8% detected after birth between the first week and the first year of life.
Of all patients with CHDs, 66.3% were born at term (between weeks 37 and 40 of gestation) and 60.6% had normal birth weights (between 2500 and 4000g); 32.0% were born preterm (gestational age <37 weeks). Within this last group, 14.9% were born before 34 weeks’ gestation and 17.1% between 34 and the 37 weeks’ gestation. Only 0.1% of patients with CHDs were born after 40 weeks’ gestation. The number of children born with low birth weights (23.5%) far exceeded the number of children born large for gestational age (6.0%). We were unable to determine the gestational age of 1.6% of the patients and the birth weight of 9.9%.
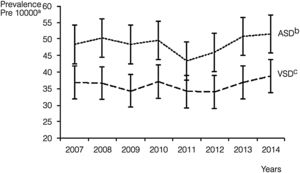
Septal malformations were the most frequent type of CHD, amounting to 55.3% of all cardiac defects. The most frequent malformations within this group were atrial septal defects (ASDs), which amounted to 29.4% of CHDs with a prevalence of 48.5 cases per 10000 births (95% CI, 46.4–50.6), followed by ventricular septal defects (VSDs), which constituted 21.8% of all CHDs with a prevalence of 36.1 cases per 10000 births (95% CI, 34.3–38.0) (Table 2). The prevalence of ASD remained between 43.4 and 51.6 cases per 10000 births. These values were higher compared to VSD, for which the peak prevalence occurred in 2014 and was of 38.8 cases per 10000 births (95% CI, 33.1, 44.6) (Fig. 2).
Distribution of congenital heart defect by type and subtype in the Valencian Region between 2007 and 2014.
| Type of congenital heart disease | ICD 10 BPAa | Cases (n) | Percentage (%) |
|---|---|---|---|
| Septal defects | Q21 | 3688 | 55.3 |
| Ventricular septal defect | Q21.0 | 1455 | 21.9 |
| Atrial septal defect | Q21.1 | 1955 | 29.3 |
| Atrioventricular septal defect | Q21.2 | 148 | 2.2 |
| Tetralogy of FALLOT | Q21.3 | 106 | 1.6 |
| Aortopulmonary septal defect | Q21.4 | 2 | 0.0 |
| Other cardiac septal malformations | Q21.5 | 15 | 0.2 |
| Cardiac septal malformation, unspecified | Q21.6 | 7 | 0.1 |
| Defects of great arteries | Q25 | 1322 | 19.8 |
| Patent ductus arteriosus | Q25.0 | 725 | 10.9 |
| Coarctation of aorta | Q25.1 | 176 | 2.6 |
| Atresia of aorta | Q25.2 | 9 | 0.1 |
| Stenosis of aorta | Q25.3 | 8 | 0.1 |
| Other aortic malformation (aplasia, hypoplasia) | Q25.4 | 100 | 1.5 |
| Atresia of pulmonary artery | Q25.5 | 8 | 0.1 |
| Stenosis of pulmonary artery | Q25.6 | 188 | 2.8 |
| Other malformation of the pulmonary artery | Q25.7 | 86 | 1.3 |
| Other malformation of great arteries | Q25.8 | 18 | 0.3 |
| Unspecified malformation of great arteries | Q25.9 | 4 | 0.1 |
| Defects of pulmonary and tricuspid valves | Q22 | 462 | 6.9 |
| Pulmonary valve atresia | Q22.0 | 18 | 0.3 |
| Pulmonary valve stenosis | Q22.1 | 181 | 2.7 |
| Pulmonary valve insufficiency | Q22.2 | 9 | 0.1 |
| Other malformation of the pulmonary valve | Q22.3 | 42 | 0.6 |
| Tricuspid valve stenosis | Q22.4 | 15 | 0.2 |
| Ebstein anomaly | Q22.5 | 13 | 0.2 |
| Hypoplastic right heart syndrome | Q22.6 | 6 | 0.1 |
| Other malformation of the tricuspid valve | Q22.8 | 177 | 2.7 |
| Uspecified malformation of the tricuspid valve | Q22.9 | 1 | <0.1 |
| Defects of mitral and aortic valves | Q23 | 417 | 6.3 |
| Aortic valve stenosis | Q23.0 | 66 | 1.0 |
| Aortic valve insufficiency | Q23.1 | 113 | 1.7 |
| Mitral valve stenosis | Q23.2 | 21 | 0.3 |
| Mitral valve insufficiency | Q23.3 | 117 | 1.8 |
| Hypoplastic left heart syndrome | Q23.4 | 39 | 0.6 |
| Other malformation of the aortic or mitral valves | Q23.8 | 59 | 0.9 |
| Uspecified malformation of the aortic or mitral valves | Q23.9 | 2 | <0.1 |
| Other cardiac defects | Q24 | 392 | 5.9 |
| Dextrocardia | Q24.0 | 27 | 0.4 |
| Laevocardia | Q24.1 | 16 | 0.3 |
| Cor triatriatum (triatrial heart) | Q24.2 | 3 | <0.1 |
| Pulmonary infundibular stenosis | Q24.3 | 8 | 0.1 |
| Subaortic stenosis | Q24.4 | 3 | <0.1 |
| Coronary artery anomalies | Q24.5 | 9 | 0.2 |
| Heart block | Q24.6 | 7 | 0.1 |
| Other congenital heart defects | Q24.8 | 61 | 0.9 |
| Unspecified congenital heart defects | Q24.9 | 258 | 3.9 |
| Defects of cardiac chambers and their connections | Q20 | 286 | 4.3 |
| Common arterial trunk | Q20.0 | 11 | 0.2 |
| Taussig-bing syndrome | Q20.1 | 20 | 0.3 |
| l-Transposition of the great vessels | Q20.2 | 6 | 0.1 |
| Discordant ventriculoarterial connection | Q20.3 | 77 | 1.2 |
| Double inlet ventricle | Q20.4 | 41 | 0.6 |
| Discordant atrioventricular connection | Q20.5 | 2 | <0.1 |
| Isomerism of atrial appendages | Q20.6 | 0 | – |
| Other defect of cardiac chambers and their connections | Q20.8 | 89 | 1.3 |
| Unspecified defect of cardiac chambers/connections | Q20.9 | 40 | 0.6 |
| Defects of great veins | Q26 | 97 | 1.5 |
| Stenosis of vena cava | Q26.0 | 2 | <0.1 |
| Persistent left superior vena cava | Q26.1 | 25 | 0.4 |
| Total anomalous pulmonary venous Connection | Q26.2 | 32 | 0.5 |
| Partial anomalous pulmonary venous connection | Q26.3 | 5 | 0.1 |
| Unspecified anomalous pulmonary venous connection | Q26.4 | 0 | – |
| Anomalous portal venous connection | Q26.5 | 2 | <0.1 |
| Hepatic arterioportal shunt | Q26.6 | 2 | <0.1 |
| Other defect of great veins | Q26.8 | 20 | 0.3 |
| Uspecified defect of the great veins | Q26.9 | 9 | 0.2 |
| Total congenital heart defects | 6664 | 100 | |
Having identified ASD as the most prevalent type of CHD, we proceeded to analyse its subtypes, that is, forms of ASD that, while all located in the atrial septum and classified under that diagnosis, present specific anatomical characteristics that allow their differentiation. The most frequent forms of ASD were patent foramen ovale and ostium secundum ASD, which amounted to 69.7% and 25.0% of atrial malformations, respectively. In addition, 0.4% of ASDs were located in the sinus venosus, 0.1% of ASDs were of yet a different type, and in 4.8% of cases, the type of ASD had not been recorded.
When we analysed maternal age, we found that CHD occurred more frequently in the group aged less than 35 years (66.7%). In our study, 31.4% of mothers were aged more than 35 years, while the age of 1.8% was unknown.
Although the maternal nationality was only recorded in some of the cases included in the study (295 out of 3671), we found a large proportion of Spanish mothers (74.2%) compared to the other frequent nationalities, including Moroccan (4.1%), Romanian (4.1%), Ecuadorian (2.7%) and Colombian (1.7%).
As for maternal health before pregnancy, 36.7% of mothers did not have a previous history of disease, while 17.9% of mothers had underlying diseases, most frequently hypothyroidism, type 1 diabetes or asthma (in 11.6%, 9.3% and 8.1% of mothers with underlying disease, respectively). We could not obtain information for this variable in 45.4% of cases.
The pregnancy was uncomplicated in 34.9% of mothers, while 17.6% received a new diagnosis of disease during pregnancy. We further divided this group of mothers into those that developed only 1 disorder during pregnancy (14.4%) as opposed to more than 1 (3.2%). There was no documentation available on this aspect in 47.5% of the cases. Gestational diabetes was the disease diagnosed most frequently during pregnancy, with 185 recorded cases, corresponding to 28.7% of the patients. It was followed by urinary tract infection, high-risk pregnancy requiring special monitoring, hypothyroidism and gestational hypertension without significant proteinuria, all of them diagnosed in more than 5.0% of women with gestational disease.
Mothers of children affected by CAs between 2007 and 2014 consumed a total of 1283 drugs. The most frequent drug was dexamethasone, a corticosteroid administered to 142 women and amounting to 11.1% of all prescribed drugs. Other drugs consumed frequently were insulin (9.2%) and levothyroxine sodium (9.0%).
According to the medical records of the children, pregnant women also received supplementation with iron (7.7%), iodine (1.9%) and vitamin complexes (1.6%). The most frequently used antibiotics were ampicillin, fosfomycin, amoxicillin or gentamycin. Other drugs used included nifedipine, methyldopa, atosiban, acetylsalicylic acid and labetalol.
Lastly, we analysed the noncardiac CAs that appeared in association with CHD in the 3671 patients in the cohort. We found 1751 associated noncardiac malformations, chiefly malformations involving the musculoskeletal system (20.1%). Of all patients with CHD, 19.2% had a syndrome, mainly Down syndrome, which amounted to 59.9% of syndromic disorders in patients with CHD. Other frequent noncardiac CAs associated with CHD involved the urinary system, nervous system, digestive system, genital system and head (eyes, ears, face and/or neck), manifesting in more than 100 patients in proportions greater than 5.0% (Table 3).
Noncardiac congenital anomalies associated with congenital heart defects in the Valencian Region between 2007 and 2014.
| Noncardiac congenital anomalies (CAs) | ICD 10 BPAa | Cases (n) | Percentage (%) |
|---|---|---|---|
| Musculoskeletal system CA | Q65–Q79 | 352 | 20.1 |
| Syndromeb | Q4471, Q6190, Q7484, Q751, Q754, Q7581, Q87, Q936, D821 Q90-Q92, Q93, Q96–Q99 | 337 | 19.2 |
| Urinary system CA | Q60–Q64 | 236 | 13.5 |
| Nervous system CA | Q00–Q07 | 183 | 10.5 |
| Digestive system CA | Q38–Q45 | 162 | 9.3 |
| Eye, ear, face and neck CA | Q10–Q18 | 127 | 7.3 |
| Genital CA | Q50–Q56 | 103 | 5.9 |
| Other CA | 251 | 14.2 | |
| Total | 1751 | 100.0 | |
The prevalence of CHDs in the VR in the period under study (91.1/10000; 95% CI, 88.1–94.0) was consistent with the estimated worldwide prevalence of CHDs (91.0/10000; 95% CI, 90.0–92.0)14 and exceeded the values published in Europe (76.1/10000; 95% CI, 75.2–77.1),4 approaching the frequency reported in Asia (93.0/10000; 95% CI, 89.0–97.0), which is the continent with the highest prevalence.
The range of prevalences observed in the VR between 2007 and 2014 (86.1/10000 [95% CI, 78.2–93.9] to 104.4/10000 [95% CI, 94.9–113.8]) was broader compared to the range registered during the same period in other regions of Spain, for instance the Basque Country (47.9/10000 [95% CI, 38.9–58.3] to 84.3/10000 [95% CI, 71.9–98.3]).15
All registries agree on septal malformations being the most frequent type of CHD, although differences emerge when the subtypes are analysed. While in Europe VSD remained the most prevalent CHD subtype (35.9/10000; 95% CI, 35.2–36.5) followed by ASD (15.3/10000; 95% CI, 14.8–15.7), in the VR this trend changed completely due to a significant increase in the prevalence of ASD (48.5/10000; 95% CI, 46.4–50.6) exceeding the prevalence of VSD (36.1/10000; 95% CI, 34.2–38.0).4,11,16
A comparison of the prevalence rates of ASD observed in the VR in the period under study (45.9/10000 [95%IC: 39.8, 52.0] to 51.6/10000 [95% CI, 45.0, 58.3]) with those recorded in the Basque Country (7.3/10000 [95% CI, 4.1, 12.0] to 17.0/10000 [95% CI, 11.9, 23.5]) where ASD was the second most frequent subtype of CHD subtype following VSD (40.6/10000; 95% CI, 33.2, 50.6) confirmed that the importance of ASD over VSD is a feature characteristic of the VC that requires further study to determine its causes.4,15 Fortunately, most of the diagnosed cases of ASD (94.7%) were cases of patent foramen ovale or ostium secundum ASD, malformations that, despite their high prevalence, are not life-threatening.17,18
Only 21.3% of cases were diagnosed prenatally, similar to the European average of 25.5%.19 In the VR, 65.6% of all patients with CHD are still diagnosed at birth, which hinders the management of the disease and treatment planning and therefore increases morbidity and mortality.20 Solving this problem requires improving the use of diagnostic techniques in our public health system, which at this time seems unattainable due to the high cost of the equipment and the need to train professionals to make adequate use of it.21
Many past studies have confirmed the association between CHDs and certain maternal diseases, such as diabetes mellitus or hypertension. Hypothyroidism, the disorder most frequently present as a chronic disease before pregnancy and the fourth most frequently diagnosed during pregnancy in our study, has also been described as a risk factor for CHD in the past,22 although in theory, hypothyroidism itself does not increase the risk of neonatal problems. The risk resides in non-adherence to treatment, which may cause preterm births associated with the development of CHDs.23,24
The substantial use of insulin, levothyroxine sodium and antibiotics is explained by the high frequency of diabetes mellitus, hypothyroidism and urinary tract infection in pregnant mothers. Nevertheless, the drug used most frequently in pregnancy was dexamethasone, which is used when there is a risk of preterm birth, an event that is particularly frequent in cases of CHD. Despite the frequent use of dexamethasone in association with CHD cases, we can rule out involvement of this drug in the development of structural CHD because corticoids are used just at the very end of pregnancy.25
A surprising finding was the low percentage of mothers that received supplementation with vitamins, iron and folic acid, as it has been shown to reduce the risk of CHD by 25.0%–50.0%, among many other benefits.26 The low frequency of supplementation found in our study was probably due to having only reviewed the medical records of the children.
There is no agreement between studies on the most frequent noncardiac CAs associated with CHD, and only Down syndrome is consistently associated with CHDs throughout the literature.27,28 Musculoskeletal, urinary or nervous system malformations, which, as evinced in our study, are very frequent in the VC, were the least frequent type in a study conducted in Turkey.29 Therefore, there may be geographical patterns in the distribution of comorbidities in CHD cases.
When it comes to the limitations of the study, we ought to highlight the lack of data for some variables that were not included in the medical records. It is our opinion that as we develop the currently existing databases, it will be necessary to create complementary information sources and improve compliance with clinical documentation to allow future research on the specific risk factors for CHD.
In conclusion, the prevalence of CHDs in the VR has been higher compared to Europe and other Spanish regions. The prevalence of ASD was higher compared to the values registered in Europe and the rest of Spain and exceeded the prevalence of VSD, which was similar to the prevalence in other regions. Among the associated maternal diseases, we ought to highlight hypothyroidism, whose relationship with CHD requires further study. The records showed that dexamethasone was the drug most frequently used during pregnancy, which can be explained by the association between the development of CHDs and preterm birth. Musculoskeletal malformations were the most frequent noncardiac CAs associated with CHDs.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Pastor-García M, Gimeno-Martos S, Zurriaga Ó, Sorlí JV, Cavero-Carbonell C. Anomalías congénitas cardíacas en la Comunitat Valenciana 2007-2014, el registro poblacional de anomalías congénitas. An Pediatr (Barc). 2020;92:13–20.