Lack of specific monitoring protocols hinders understanding of the impact of late prematurity on delayed psychomotor development. The objective of this study is to evaluate this development at 48 months and compare it with infants born at term.
Population and methodsA retrospective cohort study was conducted on 90 late preterm (LP) and 89 term (HT) healthy children at 48 months, assessed by the Ages & Stages Questionnaires® (ASQ-3). Continuous variables were analysed using the Student's t test for independent samples and described in terms of mean and standard deviation. The categorical variables were analysed using the chi-square test of independence and described in terms of frequency and proportion. ROC analysis was performed to determine the ASQ-3 cut-off value for risk of development deficit. A step-wise logistic regression model identified the associated risk factors.
ResultsThe mean scores for each domain and overall ASQ-3 score showed no differences between groups. However, when analyzing the probability density for the ASQ-3 total score of ≤251 points, 15 LP (16.6%) and 4 AT (4.5%) showed risk of psychomotor deficits, and late prematurity and lack of breastfeeding were significantly associated factors.
ConclusionsThere is an increased prevalence of risk of development deficit in LP infants, which justifies considering this population at risk and establishing effective monitoring programmes. It should be further investigated whether this risk corresponds to the entire population, or if biological factors or perinatal history can increase vulnerability.
La ausencia de protocolos específicos de seguimiento dificulta el conocimiento de la repercusión de la prematuridad tardía en el desarrollo psicomotor. El objetivo es evaluarlo a los 4 años y compararlo con los nacidos a término (AT).
Población y métodoEstudio de cohortes retrospectivo de 90 prematuros tardíos (PT) y 89 AT sanos, a los 48 meses, evaluados mediante el Ages & Stages Questionnaires® (ASQ-3). Las variables continuas se describen mediante media±DE comparadas con el test de la t de Student para muestras independientes; las variables categóricas, mediante frecuencias y proporciones, comparadas con el test de independencia de la chi al cuadrado. Se determinó un punto de corte para la puntuación total del ASQ-3 capaz de discriminar el riesgo de déficit del desarrollo mediante un análisis ROC. Una selección step-wise para el modelo de regresión logística determinó los factores de riesgo asociados.
ResultadosLas puntuaciones medias de cada dominio y de la puntuación global del ASQ-3 no mostraron diferencias entre ambos grupos. Sin embargo, al analizar la densidad de probabilidades para la puntuación global del ASQ-3≤251 puntos, 15 PT (16,6%) y 4 AT (4,5%) mostraban riesgo de déficit psicomotor, y la prematuridad tardía y la ausencia de lactancia materna resultaron factores asociados significativamente.
ConclusionesHubo una mayor prevalencia de riesgo de déficit en el desarrollo en los PT, lo que justifica considerar esta población de riesgo y establecer programas de seguimiento eficientes. Debe seguirse investigando si este riesgo corresponde a toda la población o si existen factores biológicos o antecedentes perinatales que los hacen más vulnerables.
In 2005, the vulnerability of the late preterm newborn (LP), defined as those born between 34+0 and 36+6 weeks of gestation replaced the former term “near term” that assigned a lower risk assessment to that population.1
There are numerous publications showing greater neonatal morbidity and mortality of LPs when compared to healthy term newborns.2–4
The absence of specialised follow-up programmes has made it difficult to arrive at more definitive conclusions about neurological evolution in the short and medium term. Nearly all data obtained is retrospective and with disparate results, although most indicate a greater incidence of behavioural and cognitive disorders and learning difficulties when compared to healthy term newborns.5–7
A large percentage of these children are not sufficiently evaluated after birth, and it is not known whether they present injuries associated with prematurity or secondary to neonatal morbidity. LPs are followed-up by their primary care paediatricians or family doctors, and unlike extremely premature newborns, there are no follow-up programmes specifically for this population. In the USA, 5–15% of children present developmental deficits and, in the absence of systematic evaluation tests, only 30% are diagnosed before school age.8 For this reason, the application of standardised screening scales at particular ages or due to risk factors is recommended.9 Bayley's scale10 is considered the standard for full measurement of child development, but it takes time and requires highly specialised personnel.
Questionnaires for parents and caregivers were prepared to overcome these difficulties. These instruments have been shown to be valid and reliable. The most widely used is the Ages and Stages Questionnaires® (ASQ), developed by the University of Oregon, later updated and validated in various countries,11 translated into Spanish12 and validated in Chile13,14 and Spain, in Galicia.15
Recognising the LP as a “population at risk”, would provide an easily administered and reliable tool to screen for development deficits, giving greater insight into this population and eliminating delays in prognosis and therapy.
The objective of this study was to evaluate, with a questionnaire answered by parents, the psychomotor development at 48 months of age of LPs born in our centre in 2009, and to compare them with healthy term (HT) newborns, to verify the hypothesis that LPs have twice the risk of psychomotor development difficulties than healthy term newborns.16,17
Population and methodRetrospective cohort study. LPs born in the private hospital of a health insurance company with a IIIB care level neonatal unit were enrolled.18 Two groups were established:
- –
Study group: this included premature newborns with 34+0 to 36+6 weeks of gestation, born in the hospital in 2009, and who reached the age of 48 months during 2013. Those with known malformation syndromes, genetic or metabolic diseases were excluded.
- –
Control group: this included single gestation HT newborns, between 38+0 and 41+6 weeks of age, born in the same period as the study group, and apparently healthy. Those admitted during the neonatal period and with malformation syndromes were excluded. A HT newborn was chosen for each LP included in the study, matched by date of birth.
The total number of LPs born during this time period was 155 (8.7% of live births). Their parents were contacted by telephone to explain the aim of the study and to request their participation. Of this total, 90 (57%) accepted, and the remainder either did not accept or were unreachable. The absence of significant bias between the studied LP and the excluded populations was analysed.
The control group comprised 90 HT newborns. All parents contacted by telephone agreed to take part in the study. One of them was excluded after a 42-week gestation was confirmed.
In both groups, the variables of weight at birth, gestational age measured according to obstetric data such as the date of last period and ultrasound control, gender, presence of twins, type of delivery, either vaginal or caesarean, and admission to the Neonatal Unit were recorded. In the latter case, the following parameters were recorded: respiratory pathology, hyperbilirubinaemia which required phototherapy, apnoea, hypoglycaemia (<40mg/dl in the first 48h), requirement of respiratory assistance (mechanical ventilation or CPAP) or surgery. Information about breastfeeding after 1 month of postnatal life, maternal age at the time of evaluation, academic level of the parents, single-parent family habits, and postnatal readmissions within the first year of life was gathered. The socio-economic level was deemed homogeneous (mid-high), and all parents were able to afford private health care insurance. The age at which the questionnaire was administered was noted.
The evaluation of psychomotor development was conducted by means of a questionnaire, ASQ-3 in Spanish,12 to be completed by the parents of the children at 48 months. According to the manual, it could be validly completed between 45 and 50 months and 30 days, as close as possible to 48 months.
Parents were offered the possibility to complete the questionnaire via email or during a home visit by a non-health professional who simply delivered the document and gave advice when needed. As for HT newborns, if parents preferred it, they could complete the form in the paediatrician's office or via email. They were given written information about the study, an evaluation questionnaire and an informed consent document.
Once the documents were collected and the questionnaire obtained, the parents were informed of the results via email or, in the case of detecting anomalies, they were scheduled for an office visit with the aim of providing advice on how to address the evolution of the child, following a consultation with their attending paediatrician.
The project was approved by the Ethics and Teaching Commission of the Hospital.
Evaluation instrument: the evaluation of psychomotor development was made by means of a questionnaire, ASQ-3 in Spanish12 that is easy to understand and quick to complete. There are 21 versions, adequate for each age, from 2 to 60 months. Each includes the five domains of development: communication, fine and gross motor skills, problem resolution, and sociability. In each section, six questions are raised about child performance, the answers to which were: “yes, he usually does that (10 points),” “only sometimes (5 points),” or “not yet” (0 points). The result is obtained by adding each domain and the global score. Furthermore, there are nine questions for the parents to answer directly and subjectively about some development and behavioural traits of their child. The total and partial scores are compared to the cut-off points determined by the ROC method, classifying the result as optimal, intermediate or low for the age, the latter being the average-2 SD. If at least one domain or area evaluated falls in the negative result area, more complex diagnostic tests are advisable with the aim of confirming this and starting adequate therapy.
For this study, the version of ASQ-3 at 48 months was used, attempting to have it completed as closely as possible to 48 months.
Statistical analysisTo calculate the sample size, a power of 80%, a significance of 5%, and an estimated relative risk of 2.216,17 were applied, assuming the risk rate among HT newborns to be 15%.9 A sample size of 89 children per group was obtained.
Continuous variables were described as mean±SD, and compared by means of the Student's t test for independent samples. Categorical variables were described as frequencies and proportions, and compared by means of the chi-square independence test.
ROC analysis was performed to determine the ASQ-3 cut-off value for risk of psychomotor development deficit (PDD). This point was set at 251, achieving a sensitivity of over 85.5%. Using the Kolmogorov–Smirnov test, it was determined that the distribution of probabilities of the total score was not significantly different between LP and HT for values above the cut-off point. This cut-off point was compared to the theoretical cohort point, a result obtained by subtracting 2SD from the average value of the standard sample (HT).
Two models were generated by means of step-wise logistic regression with a binary (risk/no risk) response with a retention probability of 0.5. The first model included the biodemographic/perinatal risk factors (sex, gestational age, age at completion of ASQ-3, age of mother, education of mother, single-parent habits, caesarean delivery, breastfeeding), and the second model included the clinical factors related to perinatal morbidity (neonatal admission, respiratory distress, mechanical and/or CPAP ventilation, apnoea, sepsis, hypoglycaemia, hyperbilirubinaemia, surgery, postnatal readmission).
ResultsTable 1 shows the characteristics of the study population. Notably, there is no difference between evaluated LPs and those who did not participate. Differences observed between LP and HT in weight, gestational age, presence of twins, caesarean rate, and admission to neonatology are as expected, considering the HT selection criteria. There were no differences in sex, single-parent habits and age at completion of the ASQ-3. There were differences regarding university education and breastfeeding rate, which were higher in the control group. Maternal age in the LP group was 1 year older compared to the HT group, and postnatal readmission during the first year of life was significantly higher among LPs. The age at the time of the test was 47–50 months, with an average of 48.4±1, with no differences between groups.
Sample description.
| Non-included LP | p | Study LP | p | Healthy term | |
|---|---|---|---|---|---|
| N | 66 | 90 | 89 | ||
| Weight, average±SD (g) | 2505±351.3 | NS | 2464±437.6 | <0.0001 | 3337.6±447.6 |
| GA, average±SD (weeks) | 35.6±0.52 | NS | 35.5±0.69 | <0.0001 | 39.4±1 |
| Male (%) | 33 (50) | NS | 55 (61) | NS | 49 (55) |
| Twin (%) | 30 (45) | NS | 34 (38.9) | <0.0001 | 0 |
| Caesarean (%) | 44 (66) | NS | 57 (63) | 0.004 | 39 (43.8) |
| NU admission (%) | 33 (50) | NS | 54 (60) | <0.0001 | 0 |
| Maternal university education | 69 (76.7) | 0.0485 | 76 (85.4) | ||
| Single parent (%) | 3 (3.3) | NS | 2 (2.2) | ||
| Age of the mother | 39.24±3.8 | 0.012 | 38.02±3.3 | ||
| Breastfeeding (%) | 54 (60) | 0.03 | 65 (73) | ||
| ASQ-3 age, average±SD (months) | 48.4±1 | NS | 48.3±0.8 | ||
| Postnatal readmission (%) | 12 (13.3) | 0.04 | 5 (5.6) |
ASQ-3, Ages & Stages Questionnaires; SD, standard deviation; NS, non-significant; LP, late premature; NU, Neonatal Unit; GA, gestational age.
The average scores in each of the domains and the global performance of the ASQ-3 did not show differences between LP and HT (Table 2).
ASQ-3 scores at 48 months.
| ASQ-3 score (average±SD) | Late premature (n=90) (average±SD) | Healthy term (n=89) (average±SD) | p |
|---|---|---|---|
| Communication | 57.28±6.28 | 57.92±3.97 | NS |
| Gross motor | 55.22±5.95 | 55.84±6.04 | NS |
| Fine motor | 52.22±9.18 | 54.21±7.19 | NS |
| Problem resolution | 56.33±6.66 | 57.08±5.05 | NS |
| Sociability | 55.78±5.98 | 55.78±5.59 | NS |
| Global | 275.63±24.05 | 280.17±15.93 | NS |
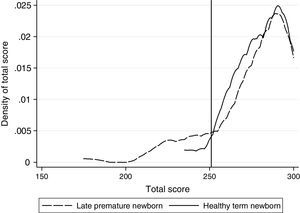
When plotting density of probabilities for the total score of ASQ-3, it was noted that both groups have an equivalent distribution starting at a total score of 251 points, and that below this point there are more LPs with low scores (Fig. 1). This cut-off point was consistent with the 2 SD-average of the HT newborn sample of reference. The ROC analysis showed a sensitivity of >85.5% for this point. The Kolmogorov–Smirnov test did not show evidence of a different distribution of probabilities between groups for scores over the cut-off point (p=0.68). Based on this analysis, a score equal to or lower than 250 was deemed a PDD risk.
Analysing the groups based on the cut-off points, 19 children appear to have PDD risk, of which 15 were LPs (16.67%) and 4 were HTs (4.5%).
In the logistic regression model, with explanatory biodemographic/perinatal variables, late prematurity (OR 3.68, CI 95%, 1.14–11.81, p=0.03) and the absence of breastfeeding (OR 3.3, CI 95%, 1.22–9.3, p=0.02) were significant. In the model with explanatory clinical variables related to perinatal morbidity, only prematurity was significant (OR 5.06, CI 95%, 1.6–15.98, p=0.006) (Table 3).
Variables significantly associated with PDD deficit risk according to the logistic regression model.
| Model 1 | Model 2 | ||
|---|---|---|---|
| Biodemographic/perinatal variables | Clinical variables | ||
| Significant variables | OR (CI 95%), p | Significant variables | OR (CI 95%), p |
| Late prematurity | 3.68 (1.14–11.81), p=0.03 | Late prematurity | 5.06 (1.6–15.98), p=0.006 |
| Absence of breastfeeding | 3.3 (1.22–9.3), p=0.02 | ||
A comparison of the global ASQ-3 score between LPs and HTs does not seem to support the initial working hypothesis. The same may be said of each of the domains explored by the questionnaire, in which no differences may be noted between groups.
However, considering the global score distribution in this sample, differences do emerge, so that LPs have higher PDD risk than HTs.
These results encourage an in-depth analysis about long-term clinical significance.
An objection might be based on the high percentage of non-participating LPs. Difficulties contacting them hindered their involvement. This was not the case with matched HTs since, with a higher number of candidates, those who were contacted and who met the inclusion criteria were enrolled. It would be desirable to have a higher number of LPs, but reaching the minimum number required by the sample calculation and the absence of differences in gestational age, weight, gender and neonatal morbidity between both groups suggest absence of bias in this respect.
Another aspect which should be considered is the characteristics of the population, with a mid-high socio-economic level and a high university education of parents, not comparable, possibly, with other studies focusing on a different social and economic population. The influence of the socio-economic level is increasingly evident, both in terms of neonatal morbidity as an independent factor19 and in long term neurological development, with PDD risk increasing when both levels are low.20,21 The results obtained contrasted with ASQ-3 scores of children with PDD in Chile at 8, 18 and 30 months.14,22
The completion of this study at 48 months is not random, since it may be considered the last step for the detection of deficits that can benefit from therapy.23 The main difficulty in the application of this questionnaire resides in the absence of validation in our country. It has been conducted in Spanish among younger children in Santiago de Chile13,14 and in Galicia.15 It is considered an excellent method for screening developmental difficulties in the USA, the Netherlands, Norway, Canada, France, Korea, Turkey, India, Iran and Brazil; in these countries, specific tests have been validated for each age, with sensitivity and specificity of around 88% and 82.5%, respectively.24,25
Considering deficits per domain decreases sensitivity and increases the specificity of the test, and therefore some authors have chosen to use global scoring as an evaluation of psychomotor development.26
Considering the characteristics of the study population, with a highly selective control group as a reference, the cut-off point is determined based on statistical criteria, thus resulting in 251 points. It was also observed that a greater number of children with PDD were found in the LP group compared to the HT control group (16.67% vs. 4.5%, respectively).
When the regression method was applied to demographic and perinatal variables, only late prematurity and the absence of breastfeeding past 1 month of life were associated with PDD; when it was applied to neonatal morbidity variables, only gestational age was significant.
For Schonhaut et al.,22 in prematures with 32–36 weeks of gestational age, gender, restricted intrauterine growth (RIG), presence of twins, and admission to neonatal intensive care (ICU) are factors probably associated with a higher risk of PDD, while for Kerstjens et al., these factors are multiple gestations, RIG, children of obese mothers, and hypoglycaemia.27,28 None of these were listed as significant in our study population.
Admission to the Neonatal Unit is associated with various hospital protocols. It would probably be more accurate as a variable for admission into neonatal ICU,29 although not everyone agrees that this is associated with poor neurological prognosis.30
The significance of absence of breastfeeding in the study population is notable. Breastfeeding is known to be a beneficial stimulant for brain development of LPs, particularly during the myelination stage.31,32 Furthermore, the difficulty of achieving successful breastfeeding among these children is well known, due to neurological immaturity, difficulties with suction and co-ordination, difficulties with maternal lactogenesis, and due to poor or no health training during the immediate neonatal period, leading to hospital readmissions due to difficulties in feeding, malnutrition, hyperbilirubinaemia and hypoglycaemia.33
Late prematurity and neonatal and postnatal morbidity appear to be significantly associated with PDD risk,19,34,35 this being greater the lower the gestational age. However, some studies have found no differences in neurological development of LPs at 12 months,36 and no conduct and learning issues during school age.37 Others, on the contrary, detect poorer school performance between 4 and 7 years among LPs.38,39
If we accept the risk of this population presenting behavioural disorders, we must be aware of the importance of having a useful, easy and reliable tool for the early detection of PDD risk. However, the problem may reside in the adaptation and validation of this questionnaire to each population, with the necessary adjustments in the cut-off points that define the standards. The need to achieve maximum sensitivity is obvious, but it is important to avoid an excessive number of false positives that would cause an emotional burden in families and a care burden for healthcare services.40
ConclusionThe follow-up study shows, in consideration of the global ASQ-3 score, a higher prevalence of PDD risk among LPs compared to HT newborns according to the criteria used. The consistency of these results should be checked to confirm their clinical significance. The characteristics of the population, as well as the absence of method validation in our country, are limitations to consider. In spite of wide consensus, obtained through multiple published studies, that LPs are at risk of presenting developmental anomalies, even as adults, it should be further investigated to determine if this statement corresponds to all the population or only to those with certain key perinatal development variables.
Conflict of interestsThe authors state there is no conflict of interest.
To Mireia Corrales, for her collaboration in the delivery, counselling, and collection of questionnaires at the homes of parents. To Gabriel Cavada, a Chilean biostatistician, for his statistical counselling. To the Private Foundation of Ms Naccari Rava (‡), a regular selfless collaborator of the hospital. To the paediatricians, Pere Catalá, Xavier Costa, Dolors Cuadra, Esperança Llorens, Inmaculada Puig and Felipe Velasco, for allowing the completion of questionnaires in their offices.
Please cite this article as: Demestre X, Schonhaut L, Morillas J, Martínez-Nadal S, Vila C, Raspall F, et al. Riesgo de déficits en el desarrollo en los prematuros tardíos: evaluación a los 48 meses mediante el Ages & Stages Questionnaires®. An Pediatr (Barc). 2016;84:39–45.