Exclusive enteral nutrition (EEN) has been shown to be more effective than corticosteroids in achieving mucosal healing without having their side effects.
ObjectivesTo determine the efficacy of EEN in terms of inducing clinical remission in newly diagnosed CD children and to study the efficacy of this therapeutic approach in improving the degree of intestinal mucosa inflammation.
Materials and methodsThe medical records of patients with newly diagnosed Crohn's disease treated with EEN were reviewed retrospectively. The degree of mucosal inflammation was assessed by fecal calprotectin (FC). Remission was defined as a PCDAI<10.
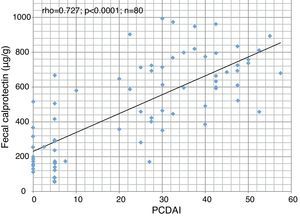
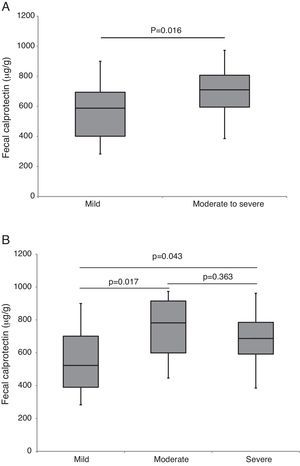
ResultsForty patients (24 males) were included, the age at diagnosis was 11.6±3.6 years. Of the 34 patients who completed the EEN period, 32 (94% per-protocol analysis) achieved clinical remission. This percentage fell to 80% in the intention-to-treat analysis. The compliance rate was 95%. Duration of EEN was 6.42 weeks (IQR 6.0–8.14). FC was significantly higher in patients with moderate and severe disease. Median baseline FC levels (680μg/g) decreased significantly to 218μg/g (p<0.0001) after EEN. We found a statistically significant correlation between FC and PCDAI (rho=0.727; p<0.0001). Early use of thiopurines (<8 weeks) vs. subsequent use was not associated with better outcome during the follow-up.
ConclusionsEEN administered for 6–8 weeks is effective for inducing clinical remission and decreasing the degree of mucosal inflammation. We did not find differences in terms of maintenance of remission in patients treated early with thiopurines.
La nutrición enteral exclusiva (NEE) ha demostrado ser más efectiva que los esteroides para alcanzar la curación mucosa sin sus efectos secundarios.
ObjetivosDeterminar la eficacia de la NEE para inducir la remisión clínica y mejorar el grado de inflamación mucosa en pacientes con EC durante su primer brote.
Material y métodosRevisión de las historias clínicas de pacientes con EC tratados con NEE durante su primer brote. El grado de inflamación mucosa se estimó mediante la calprotectina fecal (CF). Se definió remisión como PCDAI<10.
ResultadosSe incluyeron 40 pacientes (24 varones) con una edad al diagnóstico de 11,6±3,6 años. La duración de la NEE fue de 6,42 semanas (RIQ 6,0–8,14). De los 34 pacientes que completaron el período de NEE, 32 (94% en el análisis por protocolo) alcanzaron la remisión clínica. Este porcentaje descendió al 80% en el análisis por intención de tratar. La tasa de cumplimiento fue del 95%. Los valores de CF fueron significativamente mas altos en pacientes con brotes moderados y graves. La CF basal fue de 680μg/g y descendió de forma significativa a 218μg/g al final del periodo de NEE (p<0,0001). Hubo correlación estadísticamente significativa entre CF y PCDAI (rho=0,727; p<0,0001). La introducción precoz del tratamiento con tiopurinas (antes de las 8 semanas) no se asoció a una mejor evolución durante el seguimiento.
ConclusionesLa NEE administrada durante 6–8 semanas es efectiva para inducir la remisión clínica y mejorar el grado de inflamación mucosa. No encontramos diferencias en términos de mantenimiento de la remisión en pacientes tratados precozmente con tiopurinas.
Crohn's disease (CD) is an idiopathic chronic inflammatory disorder. The natural course of Crohn's disease is characterized by flare-ups alterned with periods of remission. Controlling intestinal inflammation is crucial for preventing progressive intestinal damage and the appearance of complications.1
The incidence of CD in children and adolescents is approximately 3 cases/100,000 inhabitants (range 1–8/100,000 inhab.) and this has increased in Spain and Europe during the last decade.2 Up to 20% of CD patients are diagnosed before the age of 18 years. There are differences in the form of onset, natural course, and treatment regimens between adults and children. Another key difference is delayed growth, up to 46% of children and adolescents diagnosed with CD had a decrease in their growth rate before the onset of any other symptoms, and only 12% had a normal growth rate at the time of diagnosis.3 This is not only found at diagnosis, it has a variable prevalence during follow-up.4 It is also important to remember that a chronic disease appearing during childhood is associated with considerable psychological morbidity that may influence personal relationships, psychosexual development, scholastic achievements and adherence to treatment.
There are a wide range of therapeutic objectives that need to be achieved in active disease; these include control of inflammation, mucosal healing, modifying disease progression, preventing the adverse effects of treatment and guaranteeing suitable growth and development.5,6 Exclusive enteral nutrition (EEN) has been shown to be more effective than corticosteroids, without their side effects, in achieving mucosal and transmural remission, a situation that determines better prognosis in the following years, lower hospitalization rate and less use of biological drugs.7–11
Calprotectin is a calcium-binding protein with antimicrobial properties. Calprotectin is released from the cytoplasm of activated neutrophils, and the fecal levels increase in bowel inflammation.12 Fecal calprotectin (FC) values have been correlated with endoscopic scores in adults and children with IBD. It is a non-invasive biomarker with high sensitivity and specificity that enables monitoring of inflammatory activity and prediction of clinical relapse.12–17
ObjectivesThe aims of this study were to determine the efficacy of EEN in terms of inducing clinical remission in newly diagnosed CD children and to study the efficacy of this therapeutic approach in improving the degree of intestinal mucosa inflammation using FC as a non-invasive inflammatory biomarker and to assess the effectiveness of concomitant medications. Another efficacy end point of this study was to determine whether early initiation of thiopurine treatment (before 8 weeks after diagnosis) vs. conventional introduction after relapse, improved long-term prognosis.
Materials and methodsThis was an observational retrospective study that included all patients under 14 years of age with newly diagnosed CD in a single Pediatric Gastroenterology and Nutrition Unit between January 2002 and December 2012, and who received EEN as first line therapy. The diagnosis of CD was based on established clinical, endoscopic, histological and radiological criteria.18 Patients diagnosed with ulcerative colitis, indeterminate colitis, eosinophilic colitis or infectious colitis were excluded.
All patients received a polymeric formula, Modulen IBD® or Resource IBD® (the same product with different name), both prepared the same way by mixing 1700ml water with 400g product to produce 2000ml of formula (1kcal/ml). The prescribed volumes were based on Schofield equation, which predicts resting energy expenditure.19 Feeds were gradually increased to target volumes in 3–5 days and patients were only allowed to drink water during treatment. EEN was given for a 6–8-week period.
The data collected were age, gender, family history of inflammatory bowel disease (IBD), time from diagnosis and concomitant pharmacological treatment. Disease phenotype was determined according to the Paris classification.20 All patients were assessed at the start and end of the EEN period, the variables analyzed were weight, height, body mass index (BMI), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), albumin, complete blood count (CBC), FC (Calprest®, Eurospital, Italy) and Pediatric Crohn's Disease Activity Index (PCDAI).21,22 Weight and height were measured with the patient barefoot and in underwear. Samples for the determination of fecal calprotectin were collected at home by the patient the day before and were delivered refrigerated to the laboratory for immediate analysis. Clinical remission was defined as PCDAI<10 and response as a change of more than 12.5 points from baseline PCDAI after 6–8 weeks of EEN. Normal FC levels were considered to be those below 50μg/g of feces. Weight, height and BMI z scores were calculated using data from Spanish growth charts.23
Statistical analysisVariables with normal distribution were expressed as mean±standard deviation and those without normal distribution were expressed as median and interquartile range (IQR). Kolmogorov–Smirnov test was used to evaluate normality of the distribution. Student's t-test and Wilcoxon signed-rank test were used for paired samples and Chi-square test was used to compare proportions. The estimation of correlations was performed using Spearman's Correlation Coefficient. To compare levels of FC and PCDAI a Kruskal Wallis test was applied, if the hypothesis of equality was rejected, groups were compared using the Mann Whitney test with Bonferroni correction. Survival analysis was performed with the Kaplan–Meier method. Comparison of survival curves between groups was performed with a log rank analysis. We considered a p<0.05 as statistically significant.
ResultsPatient characteristicsForty patients were included; 24 were male (60%) and the age at diagnosis was 11.6±3.6 years (Table 1). Six patients had a family history of IBD (CD in 4 and ulcerative colitis in 2). Time from the onset of symptoms to diagnosis was 4.2 months (IQR 2.3–12.1); it was slightly lower in the patients with family history of IBD (4.7 months (IQR 2.4–12.2) vs. 3.5 months (IQR 1.4–8.5), p=0.394).
Characteristics of patients treated with exclusive enteral nutrition.
| Males | 24/40 (60%) | |
| Age at diagnosis (years) | 11.6±3.6 | |
| Family history of IBD | 6/40 (15%) | |
| Time from onset to diagnosis (months) | 4.2 (IQR 2.3–12.1) | |
| Paris classification | ||
| L1 | 6 (15%) | |
| L2 | 5 (12.5%) | |
| L3 | 16 (40%) | |
| L3+L4 (extensive) | 13 (32.5%) | |
| B1 | 39 (97.5%) | |
| B2 | 1 (2.5%) | |
| Perianal (p) | 13 (32.5%) | |
| Growth retardation (G1) | 11 (27.5%) | |
| PCDAI | PCDAIa | PCDAIb |
| Mild | 11 (27.5%) | 10 (25%) |
| Moderate | * | 8 (20%) |
| Severe | 29 (72.5%) | 22 (55%) |
| Treatment when EEN was started | ||
| 5-ASA | 13 (32.5%) | |
| Metronidazole | 7 (17.5%) | |
| 5-ASA and metronidazole | 18 (45%) | |
| Metronidazole and Azithromycin | 1 (2.5%) | |
| None | 1 (2.5%) | |
PCDAI, Pediatric Crohn's Disease Activity Index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IQR, interquartile range; IBD, inflammatory bowel disease.
Baseline FC levels were 680μg/g (IQR 524–796) and decreased significantly to 218μg/g (IQR 149–402), p<0.0001 after EEN. We found a high correlation (Fig. 1) between FC and PCDAI (rho=0.727; p<0.0001). FC was significantly higher (p=0.04) in patients with moderate and severe disease (Fig. 2).
Combination of EEN with pharmacological treatmentAt the start of EEN, patients were receiving concomitant treatment with mesalazine (32.5%), metronidazole (17.5%) or both (45%) as adjuvant treatment (Table 1). Once the vaccination schedule had been checked and optimized, 27 of the 40 patients (67.5%) started treatment with azathioprine at a dose of 2.5–3.0mg/kg/day in a median time of 9 days (IQR 0–19). In two cases, azathioprine had to be suspended, one due to intolerance (abdominal pain and nausea) and another due to pancreatitis; both patients were switched to 6-mercaptopurine without incident.
Response to EENSix patients failed to complete the 6–8 weeks of EEN; two refused to continue after 2 weeks and another four received steroids after 3 weeks due to non-response. Except for one case requiring NG tube, all patients received EEN by mouth. Duration of EEN was 6.4 weeks (IQR 6–8.1). Of the 34 patients who completed the EEN period, 32 (94% per-protocol analysis) achieved clinical remission. This percentage fell to 80% in the intention-to-treat analysis. The compliance rate was 95%. There was a significant increase in weight, albumin, hemoglobin and hematocrit levels together with a significant decrease in FC, CRP, ESR, WBC and platelets (Table 2) at the end of EEN.
Clinical activities before and after EEN.
| Before EEN | After EEN | p value | |
|---|---|---|---|
| Mean weight z score | −0.68 | −0.56 | 0.042 |
| Mean height z score | −0.55 | −0.47 | 0.275 |
| Mean BMI z score | −0.09 | 0.001 | 0.024 |
| PCDAI | 40 (IQR 28–47.5) | 5 (IQR 0–5) | <0.0001 |
| FC (μg/g) | 680 (IQR 524–796) | 218 (IQR 149–402) | <0.0001 |
| CRP (mg/l) | 33.9 (IQR 16.5–67.2) | 3.1 (IQR 2.4–9.0) | <0.0001 |
| ESR (mm/h) | 29 (IQR 21.5–49) | 11 (IQR 7–16) | <0.0001 |
| Albumin (g/dl) | 3.2 (IQR 2.7–3.6) | 4.1 (IQR 3.7–4.3) | <0.0001 |
| Hemoglobin (g/dl) | 11.4 (IQR 9.9–12.3) | 12.8 (IQR 11.5–13.3) | <0.0001 |
| Hematocrit (%) | 35.2 (IQR 31.7–37.7) | 38 (IQR 35–39.7) | <0.0001 |
| WBC (×103/μL) | 10.6±3.6 | 7.7±2.6 | <0.0001 |
| Platelets (×103/μL) | 533±140 | 443±150 | <0.0001 |
PCDAI, Pediatric Crohn's Disease Activity Index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hb, hemoglobin; Hto, hematocrit; WBC, white blood cells; IQR, interquartile range; BMI, body mass index.
EEN was well tolerated in all cases and no side effects were reported during the period of use. At the end of EEN, food was introduced progressively with good acceptance.
We found no statistically significant differences in the response rate according to mild or moderate-to-severe PCDAI21 (91% vs. 76%, p=0.136), but we found differences in response rate using new PCDAI22 cut-off (90% mild, 100% moderate and 68.2% in severe CD; p=0.05). In terms of CD location, statistically significant differences in response were found (L1+L3 88% vs. L2 50% p=0.047), although only 5 patients had L2 CD location.
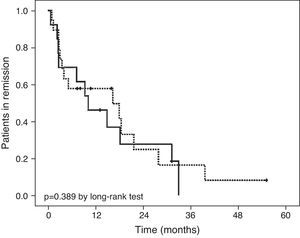
Follow-upNineteen of the 32 patients (59.4%) who achieved clinical remission had already started early (≤8 weeks after starting the EEN) treatment with azathioprine (Table 3). At the end of the follow-up period (Fig. 3), 5 patients treated with azathioprine as maintenance therapy (26.3%) and 2 with no maintenance treatment (15.3%) continued in remission (difference of 11%; 95% confidence interval, −3.13 to 25.13%; p=0.389). We did not find differences in time to relapse between groups (8.1 months (IQR 3–18) in early azathioprine group vs. 10 months (2.6–24.6) in late azathioprine group, p=0.954). The median follow-up period was 2.9 years (IQR 1.8–4.8) in both groups.
Comparison of patients between early (<8 weeks) and subsequent (>8 weeks) immunomodulator use at diagnosis and during follow-up.
| Baseline | Early IM (≤8 weeks) | Subsequent IM (>8 weeks) | p value |
|---|---|---|---|
| Number | 19 | 13 | |
| Age at diagnosis (years) | 10.9±2.8 | 11.9±3.8 | 0.910 |
| Time for diagnosis (months) | 6.4 (IQR 3.7–12.2) | 2.5 (IQR 1.4–4.1) | 0.005 |
| L1 | 4/19 (21%) | 2/13 (15%) | 0.530 |
| L2 | 1/19 (5%) | 1/13 (8%) | 0.655 |
| L3 | 14/19 (74%) | 10/13 (77%) | 0.052 |
| L4a or L4b | 9/19 (47%) | 2/13 (15%) | 0.066 |
| Growth retardation | 3/19 (16%) | 5/13 (38%) | 0.219 |
| Perianal disease | 8/19 (42%) | 3/13 (23%) | 0.233 |
| Median BMI (kg/m2) | 16.7 (IQR 15.4–18.5) | 16 (IQR 14–19.3) | 0.383 |
| Median PCDAI | 35 (IQR 27.5–42.5) | 40 (IQR 30–46.2) | 0.545 |
| Mean FC (μg/g) | 680±154 | 596±210 | 0.326 |
| Median CRP (mg/l) | 22 (IQR 16–47) | 62 (IQR 23–85) | 0.142 |
| Median ESR (mm/h) | 27 (IQR 20.7–39) | 51 (IQR 22–62) | 0.024 |
| Median Albumin (g/dl) | 3.3 (IQR 2.9–3.5) | 3 (IQR 2.4–3.9) | 0.588 |
| Mean Platelets (×103/μL) | 506±107 | 547±149 | 0.735 |
| High TPMT levels | 11/13 (84.6%) | 4/6 (66.7%) | 0.373 |
| Intermediate TPMT levels | 2/13 (15.4%) | 2/6 (33.3%) | 0.373 |
| Follow-up | |||
| Time to relapse (months) | 8.1 (3–18) | 10 (2.6–24.6) | 0.954 |
| 12 months relapse rate | 9/19 (47.3%) | 7/13 (53.8%) | 0.719 |
| 18 months relapse rate | 1/7 (14.3%) | 1/5 (20%) | 0.793 |
| 24 months relapse rate | 3/6 (50%) | 1/4 (25%) | 0.429 |
| Mean follow up (years) | 2.83 (IQR 1.1–4.7) | 2.57 (IQR 1.8–5.43) | 0.343 |
| Supplementation with enteral formula | 18/19 (94.7%) | 12/13 (92.3%) | 0.613 |
| Time receiving supplementation (months) | 12 (IQR 6.9–22.37) | 10.7 (IQR 3.5–66.9) | 0.933 |
| Prednisone treatment during follow-up | 4/19 (21%) | 11/13 (84.6%) | 0.001 |
| Change in CD behavior (B1 to B2) | 0/19 | 1/13 | 0.406 |
| IFX or ADA treatment during follow-up | 11/19 (58%) | 5/13 (38%) | 0.473 |
| Time (months) from diagnosis to starting antiTNF therapy | 15.8 (IQR 3–23) | 11 (IQR 8–57) | 0.583 |
This study shows that EEN produces clinical and biochemical remission and weight recovery in new onset CD patients. Although we did not perform an endoscopy after the EEN, the significant decrease in the FC indicates a clear improvement in the degree of intestinal inflammation.12–15 Though the rate of mucosal healing achieved by EEN in some case reports are similar to that of anti-TNF-α and much higher than steroids9–11,24–26 they suffer from bias, as EEN is more likely to be administered to patients with recent onset or mild to moderate disease, while steroids are more likely to be administered to severe disease, and anti TNF to patients with longer duration uncontrolled disease. However, unlike steroids, EEN has a positive effect on linear growth with an increase in growth rate in the 6 months following the start of treatment and on bone mineral density.27–30
In a recent study with 34 patients,11 58% of the patients treated with EEN achieved endoscopic remission (measured by SES-CD31) and 21% showed transmural remission measured by MR enterography. These authors showed that early endoscopic remission was associated with lower rates of endoscopic relapse, less use of anti-TNF-α and fewer hospitalisations one year after diagnosis. Response was better in those with less time since diagnosis. These same authors showed that induction of remission with steroids instead of EEN increased the risk of failure to respond or loss of response to anti-TNF-α.
The efficacy of EEN was first observed at the beginning of the 1970s, when it was found that the clinical and nutritional status of some patients improved after being treated with elemental formula while awaiting surgical treatment. Although the mechanisms of EEN on intestinal inflammation in CD are not fully understood, there are a number of hypotheses, including modification of the intestinal flora, elimination of the uptake of food antigens, decrease in the intestinal synthesis of inflammatory mediators due to a reduced amount of fat in the diet or the supply of micronutrients to the inflamed gut.32
Analysing the data from more than 25 pediatric studies, the overall efficacy of EEN is found to be 84% (95% CI, 81–87%), although with different enteral formulas, clinical remission criteria and treatment duration. The compliance rate is very high, reaching 90% (95% CI, 88–92%). No differences in the remission rate or compliance have been found in relation to the enteral formula used (polymeric, semi-elemental or elemental), with glutamine supplements or with medium chain triglycerides.33 In our series, only one type of formula was used, specific for CD, enriched with TGF-β, allowing no other food to be taken except water during the EEN period. Partial enteral nutrition (50% of calories in the form of conventional food) has been shown to be less effective than EEN.34 Other approaches, such as allowing up to 20% of daily estimated calories coming from other foods, have not shown lower efficacy.35
Initially it was believed that nutritional treatment was more effective in patients with ileal involvement than in those with disease located exclusively in the colon. There are currently no data to enable a correlation between response to EEN and disease phenotype.36 Moreover, in our series, we found significant differences according to the location although the number of patients with exclusive colonic disease was very small.
Although our study was not designed to address the impact of early thiopurine treatment on the outcome of the disease after induction we did not find differences in terms of maintenance of remission in patients treated early (≤8 weeks) vs. late (>8 weeks), see Table 3, but patients treated earlier needed less steroids during the follow-up (p=0.001). Our results are in concordance to other studies performed in children11,37 and in adults,38,39 and against the results showed by Markovitz et al.40 in a pediatric study. Probably one of the reasons for this discrepancy between pediatric studies may be because none of the patients included in Markovitz’40 study received induction therapy with EEN, and is already known that the achievement of mucosal healing with steroids treatment is unlikely. However, further studies are required before any evidence-based determination can be made as to the most correct use of immunomodulator therapy in pediatric CD.
This study provides valuable information on the efficacy of EEN in pediatric CD. The most important limitations are the small number of patients, which probably underestimates the effect of thiopurines and the retrospective analysis of the data, making it difficult to obtain more robust information.
In conclusion, EEN administered for 6–8 weeks is effective in inducing clinical and biochemical remission, improving anthropometric parameters and decreasing the degree of mucosal inflammation. FC enables monitoring of response to EEN and control of the degree of mucosal inflammation. Patients treated early with thiopurines received less steroids during the follow-up. The EEN period is the key for updating the vaccination schedule and ordering the necessary investigations prior to starting immunosuppression treatment.
Conflict of interestThe authors have no conflicts of interest to declare.
We thank Prof. Arie Levine MD and Prof. Julián Panés MD, PhD for their helpful contributions to this manuscript.
Please cite this article as: Navas-López VM, Blasco-Alonso J, Lacasa Maseri S, Girón Fernández-Crehuet F, Serrano Nieto MJ, Vicioso Recio MI, et al. La nutrición enteral exclusiva contínua siendo el tratamiento de primera linea en la enfermedad de Crohn pediátrica en la era de los biológicos. An Pediatr (Barc). 2015;83:47–54.