The aim of the study was to assess the use of faecal calprotectin (FCP) in infants with signs and symptoms of non-IgE-mediated cow's milk protein allergy (CMA) for both diagnosis and prediction of clinical response at the time of withdrawal of milk proteins.
Patients and methodsA one year prospective study was conducted on 82 infants between 1 and 12 months of age in the Eastern area of Málaga-Axarquía, of whom 40 of them had been diagnosed with non-IgE-mediated CMA (with suggestive symptoms and positive response to milk withdrawal), 12 not diagnosed with CMA, and 30 of them were the control group. FCP was measured at three different times: time of diagnosis, and one and three months later. ANOVA for repeated measures, nominal logistic regression and ROC curves were prepared using the SPSS.20 package and Medcalc.
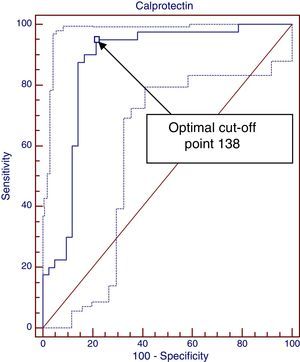
ResultsDifferences between diagnostic and control groups were assessed: there was a statistically significant relationship (P<.0001) between high FCP levels and infants suffering CMA, as well as the levels at time of diagnosis, 1 and 3 months (P<.001). A ROC curve was constructed between FCP levels and diagnosis of CMA, with 138μg/g, with the best cut-off being with an area under the curve of 0.89. However, it is only 0.68 to predict a clinical response.
ConclusionsFCP levels lower than 138μg/g could be useful to rule out non-IgE-mediated CMA diagnosis. Calprotectin is not a good test to predict clinical response to milk withdrawal.
El objetivo del estudio es evaluar la utilidad de la calprotectina fecal (CPF) en lactantes con sospecha de alergia a las proteínas de leche de vaca (APLV) no IgE mediada tanto para el diagnóstico como para predecir la respuesta clínica a la supresión láctea.
Pacientes y métodosEstudio prospectivo, de un año de duración, incluyendo 82 lactantes entre 1-12 meses en el Área Este de Málaga-Axarquía. De ellos: 40 se diagnostican de APLV no IgE mediada (síntomas compatibles y respuesta positiva a la supresión láctea), 12 no se confirma APLV y además 30 como grupo control. Se determina CPF al diagnóstico, al mes y a los 3 meses. El análisis estadístico realizado fue ANOVA para medidas repetidas, regresión logística nominal y curvas ROC utilizando los programas SPSS 20 y Medcalc.
ResultadosSe analizan diferencias entre los grupos y se objetiva relación estadísticamente significativa entre cifras elevadas de CPF y padecer APLV (p<0,0001). También se constata relación estadísticamente significativa entre cifras de CPF al diagnóstico, al mes y a los 3 meses (p<0,001). Finalmente se realiza una curva ROC entre cifras de CPF y diagnóstico de APLV resultando una área bajo la curva de 0,89 y siendo 138μg/g el mejor nivel de corte. Sin embargo, para predecir respuesta clínica este valor es únicamente de 0,68.
ConclusionesCifras de CPF inferiores a 138μg/g podrían ser útiles para descartar el diagnóstico de APLV no IgE mediada. La CPF no es un buen test para predecir respuesta clínica a la exclusión láctea.
Cow's milk protein allergy (CMPA) is a frequent problem with an estimated prevalence of 2%–7.5%.1 It is classified into immunoglobulin E (IgE)-mediated and non-IgE-mediated allergy. IgE-mediated CMPA usually manifests with cutaneous and respiratory symptoms, such as urticaria, angioedema and anaphylaxis following ingestion of cow's milk. It is diagnosed based on a thorough history taking and confirmed by means of specific IgE determination or a skin prick test and a subsequent challenge. The main symptoms in non-IgE-mediated CMPA are digestive, such as diarrhoea, vomiting, bloody stools, irritability or failure to thrive. Since allergy is not mediated by IgE in this group of patients, specific IgE or skin prick tests will not be helpful, and when it is suspected, the only diagnostic tool is the elimination of cow's milk protein from the diet for four to six weeks to watch for the resolution of symptoms. The elimination of cow's milk protein entails the use of extensively hydrolysed protein-based formulas that carry a significant economic cost and, in many instances, are rejected by the infant and the family due to their unpleasant taste and smell.1–7
Faecal calprotectin (FCP) is a protein that is mostly found in the cytoplasm of neutrophils, although it can also be expressed in the membrane of activated monocytes and macrophages. Consequently, its levels are elevated in the presence of infectious and/or inflammatory processes. In recent years, FCP has been increasingly used as a noninvasive marker for the differential diagnosis of organic and functional disease.8–12
Since the diagnosis of non-IgE-mediated CMPA is based on clinical suspicion and the elimination of cow's milk protein from the diet with evaluation of the clinical response, we decided to assess the usefulness of FCP in infants with signs and symptoms suggestive of non-IgE-mediated CMPA for the purposes of diagnosis and to predict the clinical response to a dairy-free diet.
Patients and methodsWe conducted a prospective study of one year's duration. We included 82 infants aged 1–12 months that received care at the outpatient paediatrics clinic of the Hospital de la Axarquía in the Eastern Malaga Health Management Area in Spain.
Clinical aspectsInclusion criteria: infants aged 1–12 months presenting with symptoms compatible with non-IgE mediated CMPA such as diarrhoea, vomiting, bloody stools, irritability and/or failure to thrive.1,2 We included 52 infants that presented one or more of these symptoms and were referred from Primary Care for participation in the study.
Exclusion criteria: babies aged less than 1 month or more than 12 months, IgE-mediated CMPA (verified by determination of total IgE and/or skin prick test). We also excluded patients with infectious diseases confirmed by stool or urine culture, born preterm, or with an underlying chronic disease.
Fifty-two patients met the inclusion criteria, so a sample was collected from them for FCP determination and cow's milk protein eliminated from their diets, substituting an extensively hydrolysed protein-based formula for a period of four weeks. After this period, their clinical response was evaluated by interviewing the mother, and categorised as nonexistent, poor, or good. Forty patients showed a good response, and constituted the non-IgE-mediated group of the study. Twelve patients showed no response or a poor response, and constituted the group not diagnosed with non-IgE-mediated CMPA (no CMPA). Five patients in this group were diagnosed with colic and had good clinical outcomes with no other treatment; four were diagnosed with gastroesophageal reflux, with only one of them requiring treatment with domperidone and an antacid; and the last three had weight faltering with no other diagnosis made. Last of all, we selected 30 infants aged 1 to 12 months as a control group. They were recruited from the neurologic risk clinic and were free from digestive or infectious diseases; a separate sample was collected from them for determination of FCP (Table 1).
General characteristics.
| CMPA | No CMPA | Control | |
|---|---|---|---|
| Age (mean/SD) | 3.68 mo/3.04 | 3.25 mo/1.8 | 3.8 mo/2.09 |
| Sex (M/F) | 15/25 | 6/6 | 15/15 |
| Diarrhoea | 15 | – | – |
| Rectorrhagia | 10 | – | – |
| Reflux | 29 | 4 | – |
| Weight faltering | 15 | 3 | – |
| Irritability | 19 | 5 | – |
| Total | 40a | 12 | 30 |
The determination of FCP was made at diagnosis and at one and three months following the elimination of cow's milk protein from the diet in the group diagnosed with non-IgE-mediated CMPA and the group not diagnosed with non-IgE-mediated CMPA. The followup continued until the time cow's milk could be reintroduced.
The parents or guardians of the patients gave their informed consent for their participation in the study. The study adhered to the ethical principles of the Declaration of Helsinki and those applicable to observational studies in Spain.
Laboratory methods- -
Allergy testing: to rule out IgE-mediated allergy, total IgE was determined by means of electrochemiluminescence immunoassay (ECLIA), measured in IU/mL, with normal values ranging between 0 and 100IU/mL; as well as cow's milk protein-specific IgE measured in kU/L by InmunoCAP (fluorescent enzyme immunoassay [FEIA]) with normal values corresponding to those below 0.35. Patients with a positive specific-IgE result underwent skin prick testing with 10mg/mL of purified raw cow's milk extract.
- -
Bacterial stool culture and latex agglutination tests for detection of rotavirus and adenovirus.
- -
Faecal calprotectin was determined by quantitative ELISA using Eurospital's Calprest® test, measured in μg/g and interpreted according to the following reference values: <70μg/g, negative; 70–100μg/g, borderline; and >100μg/g, positive. The test has a sensibility of 95%, a specificity of 93% and a negative predictive value (NPV) of 98%. Given the NPV of this test, false negatives are rare, but false positives are frequent, and any infectious disease, including those of the upper airways, may result in elevated FCP levels. Furthermore, the literature makes reference to slightly elevated FCP levels in preterm and term neonates aged less than 1 month with no evidence of disease.13–16
Statistical aspects: we used t tests for comparing quantitative variables, ANOVA for comparing FCP levels at diagnosis and the presence of CMPA, and repeated measures ANOVA to compare the levels of FCP at the three time points (at diagnosis, 1 month, and 3 months) and the clinical response, presence of diarrhoea, rectal bleeding, reflux, irritability and weight faltering. We used nominal logistic regression and receiver operating characteristic (ROC) curves to assess the usefulness of FCP for diagnosis and as a predictive marker of clinical response. We set the level of statistical significance for all tests at <0.05. The statistical software applications that we used were SPSS 20 (SPSS Inc, Chicago, Illinois, USA) and Medcalc 14.8.1 (MedCalc Software bvba).
ResultsWe classified the 82 patients included in the study into three groups: 40 that received a non-IgE-mediated CMPA diagnosis; 12 that did not receive a non-IgE-mediated CMPA diagnosis; and 30 in the control group. The descriptive statistics were the following: 46 patients were female (56.1%) and 36 male (43.9%). We did not find a statistically significant association between FCP values and sex (P=.946). The mean ages were 3.68, 3.25 and 3.8 months in the CMPA, no CMPA and control groups, respectively, and the differences in the mean ages were not statistically significant (P=.606).
We assessed potential differences between the mean values of FCP at diagnosis in each of the groups by means of ANOVA, and found statistically significant differences between the means of each of the analysed groups (P<.0001). Thus, the mean FCP value was 442.65μg/g in the non-IgE-mediated CMPA group, 268.58μg/g in the no CMPA group, and 100.30μg/g in the control group (Table 2).
We also compared the mean FCP values in the CPMA and the no CMPA groups by the time of determination using repeated measures ANOVA, and observed statistically significant differences (P<.001) between the FCP values at diagnosis (442.65μg/g), at one month (228.51μg/g) and at three months (92.78μg/g) from the elimination of cow's milk protein.
We also found statistically significant associations between elevated mean FCP values and the clinical manifestations of diarrhoea (P=.005; mean, 587.4μg/g) and rectal bleeding (P<.001; mean, 655.4μg/g). We did not find a significant association when we analysed reflux (P=.438; mean, 346.24μg/g), weight faltering (P=.451; mean, 371.57μg/g) or irritability (P=.469; mean, 392μg/g).
To assess the relationship between the elevation of FCP at diagnosis and non-IgE-mediated CMPA, we used nominal logistic regression and plotted a ROC curve with the Medcalc application, shown in Fig. 1. We analysed the FCP values at diagnosis comparing the CMPA group with the no CMPA group and the control group. The area under the curve was 0.889, which suggests that this is a good diagnostic test (tests are considered good between 0.75–0.9 and excellent for an area >0.9). This application offers the advantage of suggesting the cut-off point that maximises sensitivity for the highest specificity, which in this case was 138μg/g. Values greater than 138μg/g achieve a sensitivity of 95%, a specificity of 78.57%, a positive predictive value of 80.9% and a NPV of 94.3%, a positive likelihood ratio of 4.43 and a negative likelihood ratio of 0.06. It is possible to calculate the post-test probability using the positive and negative likelihood ratios and the estimated prevalence (pre-test probability of CMPA, 2%) by means of Fagan's nomogram. In this case, FCP values above 138μg/g correspond to an increased probability of having non-IgE-mediated CMPA of 8.1%, while values less than 138μg/g rule out the disease.
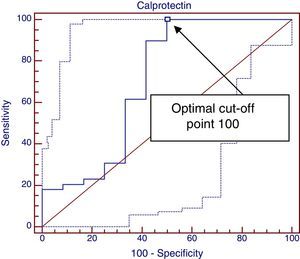
Lastly, we plotted another ROC curve to study the association between elevated FCP values at diagnosis and a positive clinical response one month after the elimination of cow's milk protein, shown in Fig. 2. We obtained an area under the curve of 0.688 (a good test corresponds to an area between 0.75 and 0.9), and the optimal cut-off point was 110, which achieved a sensitivity of 100% but had a specificity of only 50%.
DiscussionNon-IgE-mediated CMPA is a purely clinical diagnosis that is confirmed by the elimination of cow's milk protein from the diet with evidence of clinical improvement four to six weeks after initiation of the restriction diet and recurrence of symptoms upon reintroduction of milk proteins.1–5 The purpose of our study was to assess the usefulness of a simple test that could aid the decision for or against the use of cow's milk protein-free formula in case of clinical suspicion of non-IgE-mediated CMPA. This intervention carries a significant economic cost, and furthermore, in cases in which clinical suspicion is based on weight faltering, to put an example, administration of these formulas will pose some challenges due to their organoleptic characteristics.4
Faecal calprotectin has proven to be a quick, reliable and reproducible tool that facilitates the differentiation of patients with organic gastrointestinal disease from patients with functional gastrointestinal disease. Most of the literature on FCP relates to bowel inflammatory disease, a disease in which FCP has shown an excellent yield, although other studies have addressed other gastrointestinal diseases, with FCP seeming to be useful in some, such as acute gastroenteritis and gastrointestinal graft-versus-host disease, and not particularly relevant in others, such as coeliac disease and recurrent abdominal pain.17–22 In a recently published study, FCP levels were measured in children with non-IgE-mediated CMPA, and they were higher in the group that responded to the elimination of cow's milk protein than in the group that did not respond.23
Therefore, we believe that FCP could be a useful tool to assist the diagnosis of an organic gastrointestinal disease such as non-IgE-mediated CMPA, which is diagnosed exclusively on the basis of clinical findings.
The results we obtained supported our initial hypothesis, as the comparison of the mean FCP levels of the different groups under study revealed statistically significant differences, with the mean FCP being considerably higher in the non-IgE-mediated CMPA group (442.65μg/g) compared to the group that did not receive a CMPA diagnosis (268.58μg/g) and the control group (100.30μg/g).
However, when we analysed levels in relation to single presenting symptoms, we only found significant differences in patients that presented with diarrhoea or rectal bleeding, which could be explained by the increased inflammation of the digestive tract. When we analysed reflux, irritability and weight faltering we found relatively high mean values, but the differences were not statistically significant, and it is precisely in these cases that we would need additional support to make the diagnosis, as it is easier to make the decision to use hydrolysed formulas in infants that present with diarrhoea and/or rectal bleeding than in infants that present with just irritability or weight faltering. We must keep in mind that most patients presented with more than one symptom at the time of diagnosis. This may be the reason why we found FCP values above 300μg/g in some patients with reflux, irritability or weight faltering. Specifically, there were three cases in which the patients presented with rectal bleeding, irritability and weight faltering and had very elevated FCP levels (1353μg/g, 878μg/g, and 862μg/g) that contributed substantially to increases in the mean values.
We also found statistically significant differences between the FCP values measured at diagnosis and those measured at one and three months, which means that there was a significant decline in FCP levels following the elimination of cow's milk protein from the diet (441μg/g, 228μg/g, 92μg/g). This decline was concurrent with an improvement in symptoms following the elimination of milk products in the CMPA group, but the outcomes were more heterogeneous in the no CMPA group: in six patients, FCP values changed little and were always less than 100μg/g; in three, they experienced a significant decrease; while FCP values increased in another three (two patients with reflux and one with colic). These last three had good clinical outcomes, but their FCP levels normalised at a later time.
The ROC curve analysis produced interesting results. First we plotted a ROC curve with the FCP values at diagnosis in the non-IgE-mediated CMPA group, which demonstrated its value as a diagnostic test (area under the curve, 0.889; 95% CI, 0.809–0.968). We calculated the optimal cut-off point to maximise sensitivity for the best specificity, which was 138μg/g, meaning that FCP values above 138μg/g offer a sensitivity of 95% and a specificity of 78% with a positive predictive value of 80.9% and a NPV of 94%. The higher the cut-off point, the greater the specificity and the lower the sensitivity. Furthermore, we calculated the post-test probability of having CMPA by means of Fagan's nomogram, using the positive likelihood ratio (4.43) and negative likelihood ratio (0.06), and the prevalence of CMPA of around 2%. Based on our data, values higher than 138μg/g would bring the probability of having CMPA from 2% to 8.1%, and what seems more important, FCP values below 138μg/g would rule out this condition (negative post-test probability, 0.13%).
Based on the results of the second ROC curve, FCP values below 110μg/g guarantee a clinical response (sensitivity, 100%) but values above it do not guarantee a lack of response (specificity, 50%), and thus we cannot consider FCP a good marker for predicting a positive clinical response to the elimination of cow's milk protein from the diet (area under the ROC curve, 0.68; 95% CI, 0.48–0.89).
Furthermore, it is worth noting that in the control group, characterised by the absence of digestive symptoms, the mean FCP value was 109, that is, we found slightly elevated values in healthy infants. These results were consistent with other studies we reviewed, especially when FCP was measured in young babies aged less than one month or born preterm.13–16 Last of all, we need to critique our own methodology. We classified patients as belonging into the non-IgE-mediated group based solely on their clinical response four weeks after elimination of cow's milk protein, and we did not reintroduce dairy products to watch for a potential recurrence of symptoms.2 Thus, it is possible that we overestimated this diagnosis, especially in patients that presented with nonspecific symptoms, such as weight faltering and irritability. Furthermore, it is known that this disease may be temporary and resolve spontaneously within a short period of time. In any case, it is not easy to carry out the reintroduction of dairy products in everyday clinical practice, especially in infants that have experienced marked improvement.
Thus, we have concluded that: 1. FCP values below 138μg/g could be useful to rule out the diagnosis of non-IgE-mediated CMPA; 2. FCP cannot assist in the diagnosis of non-IgE-mediated CMPA in children with reflux symptoms, weight faltering and irritability, although its levels will be very elevated in children presenting with symptoms like diarrhoea and rectal bleeding; 3. FCP is not a good marker for predicting clinical response in infants with suspected non-IgE-mediated CMPA.
Conflict of interestsThe authors have no conflict of interests to declare
Please cite this article as: Trillo Belizón C, Ortega Páez E, Medina Claros AF, Rodríguez Sánchez I, Reina González A, Vera Medialdea R, et al. Calprotectina fecal como apoyo al diagnóstico en la alergia a las proteínas de leche de vaca no IgE mediada. An Pediatr (Barc). 2016;84:318–323.
Previous presentation: This paper was presented at the XXI Congreso Nacional de Gastroenterología Herpatología y Nutrición Pediátrica; May 24, 2014; Pamplona, Spain.